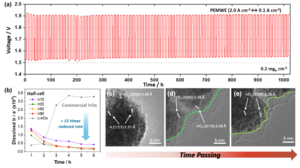
Proton exchange membrane water electrolysis (PEMWE) is considered a key technology for the climate-friendly production of hydrogen. In order to reduce production costs to the target of 1 US dollar per kilogram of hydrogen, a drastic reduction in the use of rare and expensive noble metals such as iridium is required. At the same time, the long-term stability of the catalysts must be guaranteed under industrial operating conditions.
A research team involving the Max Planck Institute for Chemical Energy Conversion has now developed a strategy to overcome this challenge. At its core is a novel, the ultrathin iridium catalyst layer whose nanostructured architecture enables exceptionally high stability and efficiency at a very low loading (only 0.2 mg/cm²).
Key to stability: Ir/IrO₂/IrOx structure
A key problem of conventional catalysts is the loss of material due to the dissolution of iridium at low loading. However, the new study shows that a controlled deposited iridium layer, only a few nanometer thin, forms a stable Ir/IrO₂/IrOx surface structure during operation. This structure counteracts the material degradation and enables constant performance over more than 1000 hours of operation.
Operando measurements with quick extended X-ray absorption fine structure (quick EXAFS), identical location transmission electron microscopy (IL-TEM) and other analytical methods provided insights into the formation and stability of this catalyst structure.
Precise control on the nanoscale
The catalytically active layer was produced in a targeted manner in the nanometer range using an advanced electrochemical deposition technology – a significant advance over conventional micrometer-thick catalyst layers. The key is the limited growth of an amorphous IrOx layer that protects a stable crystalline underlayer of Ir/IrO₂ – and thus contributes to the exceptional longevity of the catalyst.
Significance for practical applications
The work demonstrates the stable long-term operation of a PEM electrolyzer at industrially relevant current densities (2 A/cm²) with an extremely low iridium loading – at a minimal degradation rate of only 8.7 µV/h. The study thus provides an important contribution to the development of cost-efficient and long-lasting electrode catalysts for large-scale hydrogen production.
Several scientists from the Max Planck Institute for Chemical Energy Conversion were significantly involved in the study, including Ahyoun Lim (first author of the paper and postdoc in the Electrochemistry Group), Marc Tesch (postdoc in the Electrochemistry Group), Daniela Ramermann (postdoc in the group Electron Microscopy & XPS Group) Walid Hetaba (group leader of the Electron Microscopy & XPS Group) and Ioannis Spanos (group leader of the Electrochemistry Group).
The results were published in the renowned journal ACS Catalysis:
Original publication:
Ahyoun Lim et al., Limited surface oxide growth as a prerequisite for stabilizing low-loading iridium electrodes for PEM water electrolysis,
ACS Catalysis, 2025, 15, 6098–6113
DOI: 10.1021/acscatal.4c07864