Dr. Ioannis Spanos - Electrochemistry
- Dr. Ioannis Spanos
- Group leader
- Electrochemistry
- Heterogeneous Reactions
- +49 (0)208 306 - 3845
- ioannis.spanos(at)cec.mpg.de
- Room: 672
Vita
| BSc. Physics | University of Patras, Greece (2006) | ||
| M.Sc. Environmental Sciences | University of Patras, Greece (2009) | ||
| Research Assistant | University of Patras, Greece (2009-2011) | ||
| Ph.D in Nanochemistry | University of Copenhagen, Denmark (2014) | ||
| Research Assistant | University of Copenhagen, Denmark (2014-2015) | ||
| Postdoc | MPI CEC (2015-2020) | ||
| Group leader | MPI CEC (seit 2020) | ||
Publications
Full publications list | ORCID | ResearcherID | Google Scholar Profile
Selected MPI CEC publications
- Lim, A., Tesch, M. F., Spanos, I. (2023). The power of operando analysis: Understanding the critical characteristics of OER catalysts from atomistic to systemic scales. CURRENT OPINION IN ELECTROCHEMISTRY, (39): 101272, pp. 1-10. doi:10.1016/j.coelec.2023.101272.
- Papakonstantinou, G., Spanos, I., Dam, A. P., Schlögl, R., Sundmacher, K. (2022). Electrochemical evaluation of the de-/re-activation of oxygen evolving Ir oxide. Phys. Chem. Chem. Phys., 24, 14579-14591.https://doi.org/10.1039/D2CP00828A
- Haase, F. T., Rabe, A., Schmidt, F.-P., Herzog, A., Jeon, H. S. Jeon, Frandsen, W., Narangoda, P. V., Spanos, I., Ortega, K. F., Timoshenko, J., Lunkenbein, T., Behrens, M., Bergmann, A., Schlögl, R., and Roldan Cuenya, B. (2022). Role of Nanoscale Inhomogeneities in Co2FeO4 Catalysts during the Oxygen Evolution Reaction. Journal of the American Chemical Society. 144 (27), 12007-12019 https://pubs.acs.org/doi/10.1021/jacs.2c00850
- Spanos, I., Masa, J., Zeradjanin, A., Schlögl, R. (2020). The Effect of Iron Impurities on Transition Metal Catalysts for the Oxygen Evolution Reaction in Alkaline Environment: Activity Mediators or Active Sites? Catalysis Letters. https://doi.org/10.1007/s10562-020-03478-4
- Ruiz Esquius, J., Algara-Siller, G., Spanos, I., Freakley, S.J., Schlögl, R., Hutchings, G.J. (2020). Preparation of Solid Solution and Layered IrOx–Ni(OH)2 Oxygen Evolution Catalysts: Toward Optimizing Iridium Efficiency for OER ACS Catalysis 10(24), 14640-14648. https://doi.org/10.1021/acscatal.0c03866
- Zeradjanin, A.R., Spanos, I., Masa, J., Rohwerder, M., Schlögl, R. (2020). Perspective on experimental evaluation of adsorption energies at solid/liquid interfaces Journal of Solid State Electrochemistry. https://doi.org/10.1007/s10008-020-04815-8
- Antonyshyn, I., Barrios Jiménez, A.M., Sichevych, O., Burkhardt, U., Veremchuk, I., Schmidt, M., Ormeci, A., Spanos, I., Tarasov, A., Teschner, D., Algara-Siller, G., Schlögl, R., Grin, Y. (2020). Al2Pt for Oxygen Evolution in Water Splitting: a Strategy for Creating Multi‐functionality in Electrocatalysis Angewandte Chemie International Edition 59(38), 16770-16776. https://doi.org/10.1002/anie.202005445
- Ding, Y., Gu, Q., Klyushin, A., Huang, X., Choudhury, S.H., Spanos, I., Song, F., Mom, R., Düngen, P., Mechler, A.K., Schlögl, R., Heumann, S. (2020). Dynamic carbon surface chemistry: revealing the role of carbon in electrolytic water oxidation Journal of Energy Chemistry 47, 155-159. https://doi.org/10.1016/j.jechem.2019.12.006
- Ruiz Esquius, J., Morgan, D.J., Spanos, I., Hewes, D.G., Freakley, S.J., Hutchings, G.J. (2020). The effect of Base on the Facile Hydrothermal Preparation of Highly Active IrOx Oxygen Evolution Catalysts ACS Applied Energy Materials 3(1), 800-809. https://doi.org/10.1021/acsaem.9b01642
- Spanos, I., Tesch, M.F., Yu, M., Tüysüz, H., Zhang, J., Feng, X., Müllen, K., Schlögl, R., Mechler, A.K. (2019). Facile protocol for alkaline electrolyte purification and its influence on a Ni-Co oxide catalyst for the oxygen evolution reaction ACS Catalysis 9(9), 8165-8170. https://doi.org/10.1021/acscatal.9b01940
- Rodenas, T., Beeg, S., Spanos, I., Neugebauer, S., Girgsdies, F., Algara-Siller, G., Schleker, P.P.M., Jakes, P., Pfänder, N., Willinger, M., Greiner, M., Prieto, G., Schlögl, R., Heumann, S. (2018). 2D Metal Organic Framework-Graphitic Carbon Nanocomposites as Precursors for High-Performance O2-Evolution Electrocatalysts Advanced Energy Materials 8(35), 1802404. https://doi.org/10.1002/aenm.201802404
- Spanos, I., Neugebauer, S., Guterman, R., Yuan, J., Schlögl, R., Antonietti, M. (2018). Poly(ionic liquid) Binders as Ion conductors and Polymer Electrolyte Interface for Enhanced Electrochemical Performance of Water Splitting Electrodes Sustainable Energy & Fuels 2(7), 1446-1451. https://doi.org/10.1039/C8SE00110C
- Düngen, P., Greiner, M., Böhm, K.H., Spanos, I., Huang, X., Auer, A.A., Schlögl, R., Heumann, S. (2018). Atomically dispersed vanadium oxides on multiwalled carbon nanotubes via atomic layer deposition: A multiparameter optimization Journal of Vacuum Science & Technology A 36(1), 01A126. https://doi.org/10.1116/1.5006783
- Spanos, I., Auer, A.A., Neugebauer, S., Deng, X.H., Tüysüz, H., Schlögl, R. (2017). Standardized Benchmarking of Water Splitting Catalysts in a Combined Electrochemical Flow Cell/Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES) Setup ACS Catalysis 7(6), 3768-3778. https://doi.org/10.1021/acscatal.7b00632
- Auer A.A., Cap S., Antonietti M., Cherevko S., Deng X., Papakonstantinou G., Sundmacher K., Brüller S., Antonyshyn I., Dimitratos N., Davis R.J., Böhm K.-H., Fechler N., Freakley S., Grin Y., Gunnoe B.T., Haj-Hariri H., Hutchings G., Liang H., Mayrhofer K.J.J., Müllen K., Neese F., Ranjan C., Sankar M., Schlögl R., Schüth F., Spanos I., Stratmann M., Tüysüz H., Vidakovic-Koch T., Yi Y., Zangari G. (2016). MAXNET Energy - Focusing Research in Chemical Energy Conversion on the Electrocatlytic Oxygen Evolution Green 5(1-6), 7-21. http://doi.org/10.1515/green-2015-0021
Research 'Electrochemistry'
The aim of our research is the investigation of dynamic energy conversion phenomena at electrified interfaces, with main focus on water electrolysis (i.e. oxygen evolution reaction (OER) and hydrogen evolution reaction (HER)) as well as fuel cell reactions (particular focus on oxygen reduction reaction (ORR)). The in-depth investigation of such complex phenomena requires a multidisciplinary approach. Therefore, we employ methodologies that will address scientific challenges from various relevant perspectives. This kind of multifaceted approach can give more complete and in-depth knowledge on the underlying electrocatalytic mechanisms. For this reason, a variety of different metal-based, metal oxide-based or composite-based catalytic systems (flat samples, nanostructured samples, polycrystalline materials, single crystals etc..) are evaluated under transient and steady state conditions using advanced analytics including: Inductively Coupled Plasma – Optical Emission Spectroscopy (ICP-OES) coupled to electrochemical Quartz Crystal Microbalance (eQCM), elevated temperature Rotating Disc Electrode (RDE), Kelvin probe (KP) etc. Gathered information on complex behavior of electrified interfaces during electrocatalytic performance (e.g. intrinsic rate of dissolution, transient mass changes, effect of impurities on rate of electrocatalytic reaction, adsorption energies etc.) will give us an advanced view on electrocatalytic processes and supply valuable information about the properties essential to identify electrocatalysts with superior performance. Therefore, our research activities are divided into sub-divisions which mutually interact and complement each other: 1) Analytical Electrochemistry (Dr. Ioannis Spanos, Group Head) 2) Physical Electrochemistry (Dr. Aleksandar Zeradjanin) and 3) Materials Electrochemistry (Dr. Justus Masa).
Results of complementary research interactions are knowledge-guided principles of electrocatalyst and electrode design for electrochemical energy systems. A key prerequisite to successful electrocatalyst and electrode design is a solid understanding of material and interface properties prior to electrochemical application, but most importantly, under electrochemical reaction conditions, as a basis for deriving structure-activity relationships. Beyond understanding of materials and interfaces as collective entities, our research also seeks to understand complexity of electrocatalytic process on atomic and molecular level by resolving the nature of active catalytic site(s) under dynamic reaction conditions where we employ advanced operando spectroscopic techniques (e.g. EXAFS, Raman, ATR-FTIR etc…). Therefore, of essential importance is understanding of how to activate water splitting in electrolyzers (or water formation in fuel cells) will be experimental and theoretical studies on electron and ion transfer at electrocatalytic interfaces in order to extract relevant material-based and interface-based catalytic descriptors. Besides intrinsic properties of electrocatalysts, we study various electrode structures in order to understand how to accelerate mass transport at, if possible, technical conditions. In that context, the spatio-temporal analysis of gas-evolving electrocatalytic electrodes (e.g. by using SECM), can help significantly in finding a pathway how to meaningfully enhance gas evolution including the mechanisms of degradation of metals/oxides due to local extreme conditions of overpotential, pH etc.
In summary, our research thus entails a multi-faceted research approach that includes: 1) development of analytical tools and techniques for in-situ and in-operando catalyst characterization to understand dynamic properties of the catalyst under reaction conditions, consequently improving the theoretical and experimental framework for better interpretation of catalyst properties and performance; 2) expanding boundaries of the fundamental knowledge about electrocatalytic energy conversion at electrified interfaces, including new facets in understanding of interfacial charge transfer as well as the development of catalytic descriptors and quantitative structure-activity correlations; 3) knowledge-based catalyst design.
Below are illustrated few research points of contemporary interest.
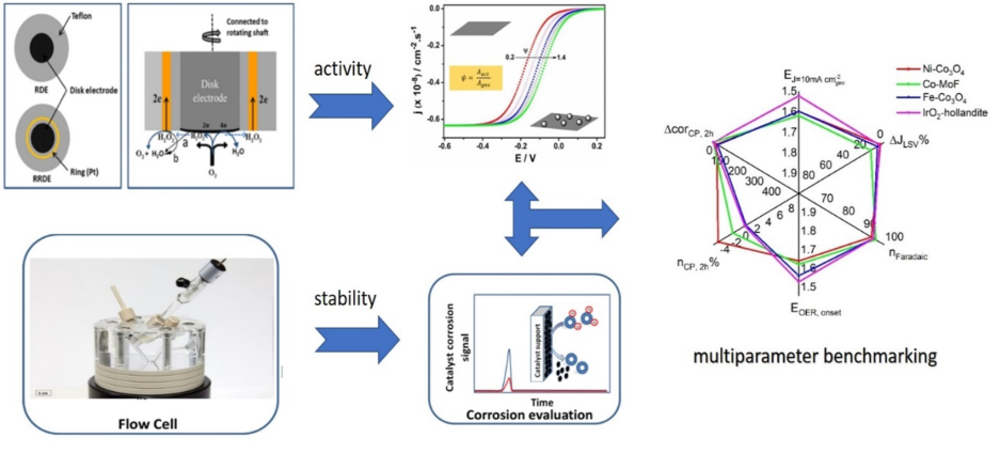
Advanced protocols for electrocatalyst activity/stability benchmarking
While importance of activity/stability benchmarking is widely accepted, most of the research groups that strive nowadays to establish rigid benchmarking protocols, are focused on activity due to the widely accepted usage of RDE and due to lack of suitable analytical tools for measuring of in-operando catalyst dissolution. In our lab, besides classical kinetic tools like RDE, several designs of flow cells coupled to ICP-OES were developed, allowing potential-dependent time resolved monitoring of catalyst dissolution. All obtained results relevant for benchmarking protocols can be illustrated with one “spider” graph as shown in Fig.2.
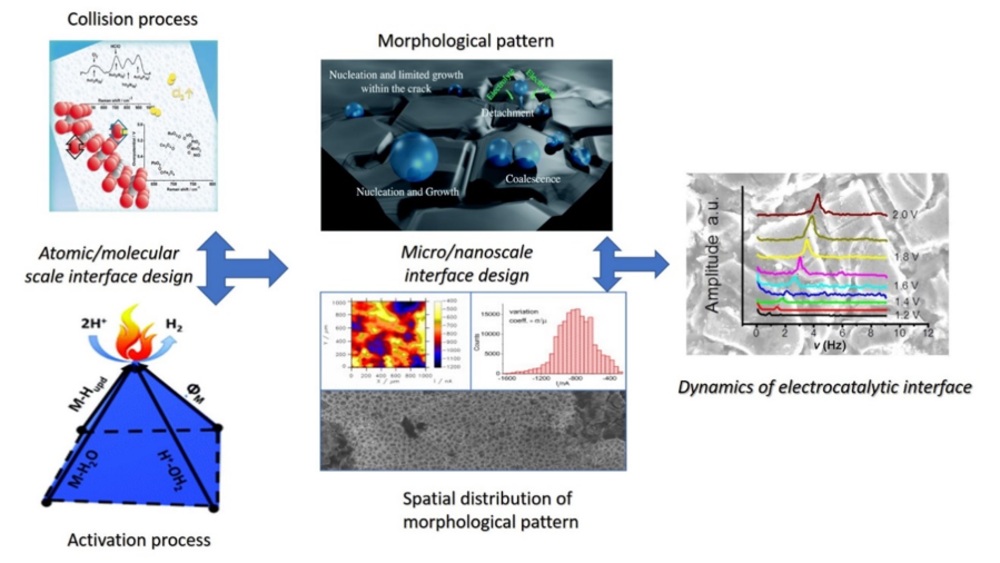
Development of relevant electrocatalytic descriptors
One of the essential tasks of our research group is to identify physicochemical properties of catalytic materials and properties of electrode/electrolyte interfaces, that can give correlation to the reaction rate. These properties given in a quantitated form as parameters are called catalytic descriptors. They are of essential importance when it comes to understanding not only the mechanisms of electrocatalytic processes, but also in understanding the functionality of morphological patterns. At the same time catalytic descriptors are the main foundation of rational catalyst design.
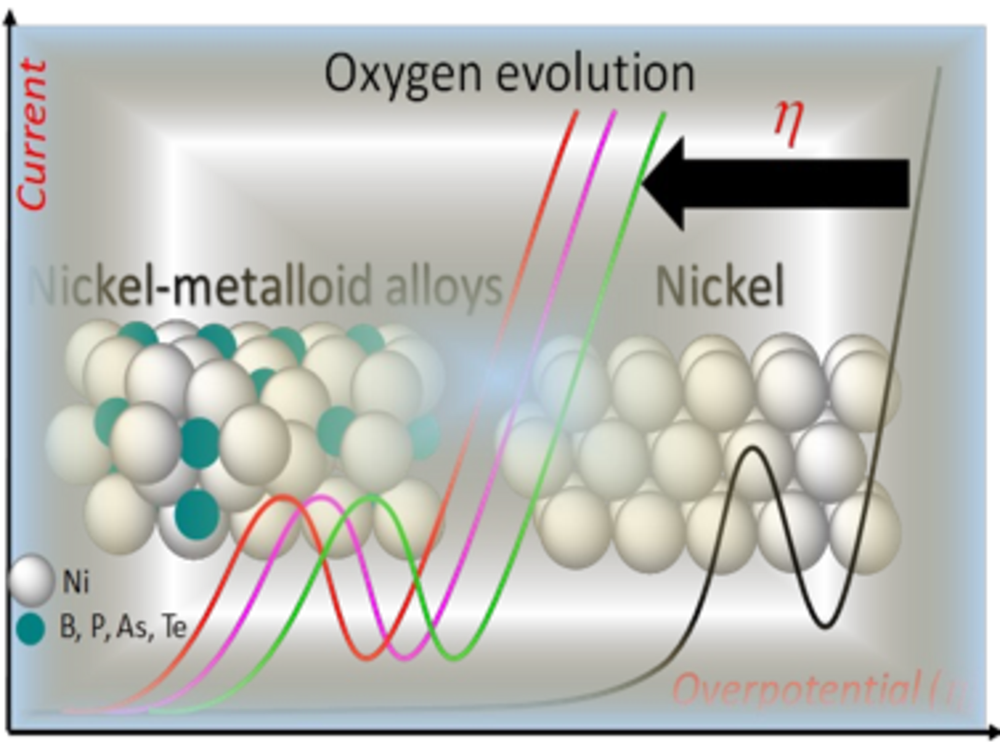
Complex materials in electrocatalysis (e.g. metal-metalloid alloys)
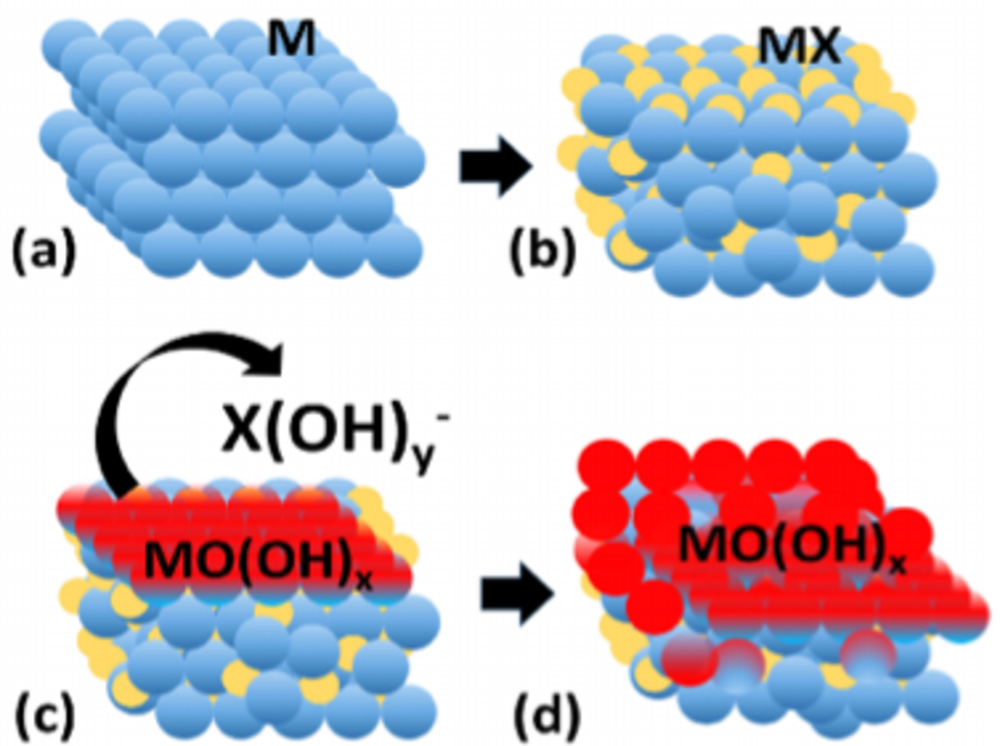
Metal-metalloid (MX) alloys exhibit intriguing properties that combine metallic, covalent and ionic character due to their unique M-M, M-X and X-X bonding. Metal-metalloid alloys with predominant metallic properties show interesting electrocatalytic properties and have been used in electrocatalytic water splitting. The research interests of the group are to decipher the electronic and geometric properties of metal-metalloid electrocatalysts that determine their electrocatalytic properties with the ultimate goal of using such model structures and the underlying properties to design improved catalysts.
Elucidation of the electrochemical and chemical mechanisms of transition from metal-metalloid (MX) pre-catalysts to MX@(MX)OxH core@shell structures, understanding the function of the metalloid elements in activity and stability enhancement and the influence of dissolved metalloid oxo-anions on the interaction energies of reaction intermediates in the electrochemical double layer and how they influence reaction mechanisms, activity and stability, are some of the specific research objectives of the group. The ultimate goal is the translation of the fundamental cross-cutting knowledge gained from such in-depth studies into general catalyst design concepts to realize more efficient catalysts of industrial relevance.
References
[1] Aleksandar R. Zeradjanin, George Polymeros, Cigdem Toparli, Marc Ledendecker, Nejc Hodnik, Andreas Erbe, Michael Rohwerder and Fabio La Mantia. What is the trigger for the hydrogen evolution reaction?–towards electrocatalysis beyond the Sabatier principle. PCCP 22, 8768-8780, 2020 https://doi.org/10.1039/D0CP01108H
[2] Aleksandar R. Zeradjanin Is a major breakthrough in the oxygen electrocatalysis possible?. Current Opinion in Electrochemistry 9, 214-223, 2018 https://doi.org/10.1016/j.coelec.2018.04.006
[3] Aleksandar R Zeradjanin, Edgar Ventosa, Justus Masa, Wolfgang Schuhmann. Utilization of the catalyst layer of dimensionally stable anodes. Part 2: Impact of spatial current distribution on electrocatalytic performance. Journal of Electroanalytical Chemistry 828, 63-70, 2018 https://doi.org/10.1016/j.jelechem.2018.09.034
[4] Aleksandar R. Zeradjanin Frequent Pitfalls in the Characterization of Electrodes Designed for Electrochemical Energy Conversion and Storage. ChemSusChem, 11, 1-8, 2018 https://doi.org/10.1002/cssc.201702287
[5] Aleksandar R. Zeradjanin, Ashokanand Vimalanandan, George Polymeros, Angel A. Topalov, Karl J.J. Mayrhofer, Michael Rohwerder. Balanced work function as a driver for facile hydrogen evolution reaction – comprehension and experimental assessment of interfacial catalytic descriptor. PCCP, 19, 17019-17027, 2017 https://doi.org/10.1039/C7CP03081A
[6] Masa, J., Andronescu, C., Schuhmann, W. (2020). Electrocatalysis as the nexus for sustainable renewable energy. The Gordian knot of activity, stability, and selectivity. Angewandte Chemie International Edition https://doi.org/10.1002/anie.202007672
[7] Wilde, P., Dieckhöfer, S., Quast, T., Xiang, W., Bhatt, A., Chen, Y.-T., Seisel, S., Barwe, S., Andronescu, C., Li, T., Schuhmann, W., Masa, J. (2020). Insights into the formation, chemical stability and activity of transient NiyP@NiOx core-shell heterostructures for the oxygen evolution reaction ACS Applied Energy Materials 3(3), 2304-2309. https://doi.org/10.1021/acsaem.9b02481
[8] Morales, D.M., Kazakova, M.A., Purcel, M., Masa, J., Schuhmann, W. (2020). The sum is more than its parts: stability of MnFe oxide nanoparticles supported on oxygen-functionalized multi-walled carbon nanotubes at alternating oxygen reduction reaction and oxygen evolution reaction conditions Journal of Solid State Electrochemistry https://doi.org/10.1007/s10008-020-04667-2
[9] Krysiak, O.A., Junqueira, J.R.C., Conzuelo, F., Bobrowski, T., Masa, J., Wysmolek, A., Schumann, W. (2020). Importance of catalyst–photoabsorber interface design configuration on the performance of Mo-doped BiVO4 water splitting photoanodes Journal of Solid State Electrochemistry https://doi.org/10.1007/s10008-020-04636-9
[10] Masa, J., Barwe, S., Andronescu, C., Schuhmann, W (2018). On the Theory of Electrolytic Dissociation, the Greenhouse Effect, and Activation Energy in (Electro) Catalysis: A Tribute to Svante Augustus Arrhenius. Chemistry A European Journal 25 (1), 158-166. https://doi.org/10.1002/chem.201805264
[11] Spanos I., Tesch M. F., Yu M., Tüysüz H., Zhang J., Feng X., Müllen K., Schlögl R. and Mechler A. K., ‘A facile protocol for alkaline electrolyte purification and its influence on a Ni-Co oxide catalyst for the oxygen evolution reaction’, ACS Catal. 2019, 9, 9, 8165-8170. https://doi.org/10.1021/acscatal.9b01940
[12] Spanos I., Neugebauer S., Guterman R., Yuan J., Schlögl R. and Antonietti M., ‘Poly(ionic liquid) binders as ionic conductors and polymer electrolyte interfaces for enhanced electrochemical performance of water splitting electrodes’, Sustainable Energy Fuels, 2018, 2, 1446. https://doi.org/10.1039/C8SE00110C
[13] Spanos I., Auer A.A., Neugebauer S., Deng X., Tüysüz H., and Schlögl R., ‘Standardized benchmarking of water splitting catalysts in a combined electrochemical flow cell/ICP-OES setup’, ACS Catal. 2017, 7, 3768-3778. https://doi.org/10.1021/acscatal.7b00632