Dr. Walid Hetaba - Electron Microscopy & XPS
- Dr. Walid Hetaba
- Group leader
- Electron Microscopy & XPS
- Heterogeneous Reactions
- +49 (0)208 306 - 3746
- walid.hetaba(at)cec.mpg.de
- Room: A 02.006
Vita
| Diplom | TU Wien, Dipl.-Ing. (2011) |
| PhD | TU Wien, Dr.techn. (2011-2015) |
| Scientific Staff | TU Wien (2011-2015) Universität Bielefeld (2013-2014) |
| PostDoc | Fritz-Haber-Institut der MPG and MPI CEC (2016-2020) |
| Visiting Scientist | Humboldt-Universität zu Berlin, WG Structural Research/Electron Microscopy (since 2018) |
| Group leader | MPI CEC (since 2020) |
Publications
Full publications list | ORCID | ResearcherID | Google Scholar Profile | Scopus Author ID
Selected MPI CEC publications
- Hetaba, W., Imlau, R., Duarte-Correa, L., Lamoth, M., Kujawa, S., & Lunkenbein, T. (2021). ChemiTEM – Transmission Electron Microscopy Optimized for Chemistry and Material Science. Chemistry–Methods, 1(9), 401-407. https://doi.org/10.1002/cmtd.202100001
- Lin, S.-H., Hetaba, W., Chaudret, B., Leitner, W., Bordet, A. (2022). Copper-Decorated Iron Carbide Nanoparticles Heated by Magnetic Induction as Adaptive Multifunctional Catalysts for the Selective Hydrodeoxygenation of Aldehydes. Advanced Energy Materials,12(42) 2201783, pp. 1-10. doi:10.1002/aenm.202201783.
- Löffler, S., Stöger-Pollach, M., Steiger-Thirsfeld, A., Hetaba, W., Schattschneider, P. (2021). Exploiting the Acceleration Voltage Dependence of EMCD. Materials, 14(5): 1314, pp. 1-14. doi:10.3390/ma14051314.
- Gioria, E., Duarte-Correa, L., Bashiri, N., Hetaba, W., Schomaeker, R., Thomas, A. (2021). Rational design of tandem catalysts using a core-shell structure approach. Nanoscale Advances, 3(12), 3454-3459. doi:10.1039/d1na00310k.
- Masliuk, L., Schmidt, F.-P., Hetaba, W., Plodinec, M., Auffermann, G., Hermann, K., Teschner, D., Girgsdies, F., Trunschke, A., Schlögl, R., Lunkenbein, T. (2020). Compositional Decoupling of Bulk and Surface in Open-Structured Complex Mixed Oxides The Journal of Physical Chemistry C 124(42), 21069-23077. https://doi.org/10.1021/acs.jpcc.0c04777
- Hetaba, W., Klyushin, A.Y., Falling, L.J., Shin, D., Mechler, A.K., Willinger, M.-G., Schlögl, R. (2020). Investigation of Electrocatalysts Produced by a Novel Thermal Spray Deposition Method Materials 13(12), 2746. https://doi.org/10.3390/ma13122746
- Koch, G., Hävecker, M., Teschner, D., Carey, S.J., Wang, Y., Kube, P., Hetaba, W., Lunkenbein, T., Auffermann, G., Timpe, O., Rosowski, F., Schlögl, R., Trunschke, A. (2020). Surface Conditions that Constrain Alkane Oxidation on Perovskites ACS Catalysis 10(13), 7007-7020. https://doi.org/10.1021/acscatal.0c01289
- Wolf, E.H., Millet, M.-M., Seitz, F., Redeker, F.A., Riedel, W., Scholz, G., Hetaba, W., Teschner, D., Wrabetz, S., Girgsdies, F., Klyushin, A., Risse, T., Riedel, S., Frei, E. (2020). F-doping of nanostructured ZnO: A way to modify structural, electronic, and surface properties Physical Chemistry Chemical Physics 22(20), 11273-11285. https://doi.org/10.1039/D0CP00545B
- El Sayed, S., Bordet, A., Weidenthaler, C., Hetaba, W., Luska, K., Leitner, W. (2020) Selective Hydrogenation of Benzofurans using Lewis Acid Modified Ruthenium-SILP Catalysts ACS Catalysis 10(3), 2124-2130. https://doi.org/10.1021/acscatal.9b05124
- Häusler, I., Kamachali, R.D., Hetaba, W., Skrotzki, B. (2019). Thickening of T1 Precipitates during Aging of a High Purity Al–4Cu–1Li–0.25Mn Alloy Materials 12(1), 30. https://doi.org/10.3390/ma12010030
- Straten, J.W., Schlecker., P., Krasowska, M., Veroutis, E., Granwehr, J., Auer, A.A., Hetaba, W., Becker, S., Schlögl, R., Heumann, S. (2018). N-Funtionalized Hydrothermal Carbon Materials using Urotropine as N-Precursor Chemistry - A European Journal 24(47), 12298-12317. https://doi.org/10.1002/chem.201800341
- Anke, B., Rohloff, M., Willinger, M.G., Hetaba, W., Fischer, A., Lerch, M. (2017). Improved photoelectrochemical performance of bismuth vanadate by partial O/F-substitution Solid State Sciences 63, 1-8. https://doi.org/10.1016/j.solidstatesciences.2016.11.004
- Häusler, I., Schwarze, C., Bilal, M.U., Ramirez, D.V., Hetaba, W., Kamachali, R.D., Skrotzki, B. (2017). Precipitation of T1 and θ′ Phase in Al-4Cu-1Li-0.25Mn During Age Hardening: Microstructural Investigation and Phase-Field Simulation Materials 10(2), 117. https://doi.org/10.3390/ma10020117
- Rudi, S., Teschner, D., Beermann, V., Hetaba, W., Can, L., Cui, C., Gliech, M., Schlögl, R., Strasser, P. (2017). pH-Induced versus Oxygen-Induced Surface Enrichment and Segregation Effects in Pt Ni Alloy Nanoparticle Fuel Cell Catalysts ACS Catalysis 7(9), 6376-6384. https://doi.org/10.1021/acscatal.7b00996
- Hetaba, W., Stöger-Pollach, M. (2016). EMCD investigation of the Verwey‐transition in magnetite European Microscopy Congress 2016: Proceedings 1086-1087. https://doi.org/10.1002/9783527808465.EMC2016.6656
Group members
Electron microscopy & XPS
In the electron microscopy and XPS group, we investigate materials at micro- and nanoscale up until atomic resolution. Using our spectroscopic methods, we gain information about the chemical composition and the electronic structure of the studied samples. By investigating catalyst samples using these methods we aim to relate the structure and function of the materials. This helps with the development of improved catalysts and processes.
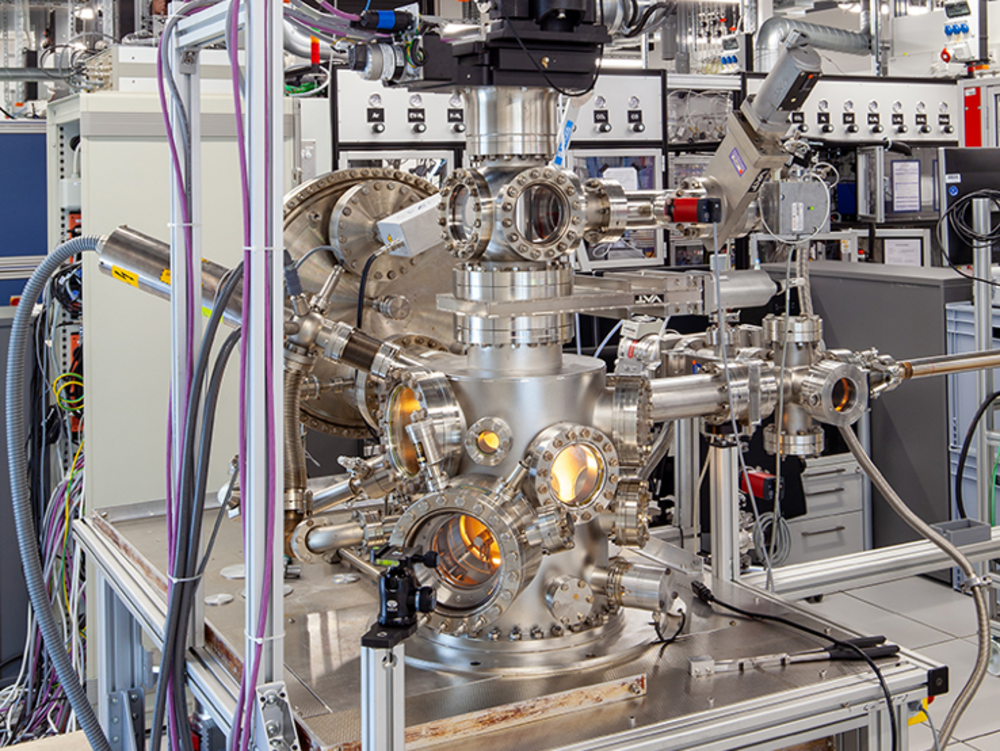
Transmission electron microscopy (TEM) is a versatile method as it allows the use of a wide variety of different techniques in a single instrument (Fig. 1a). Not only can we image the investigated samples at high resolution but also perform electron diffraction experiments. These techniques can be combined with analytical investigations using energy dispersive X-ray spectroscopy (EDS) and electron energy-loss spectrometry (EELS). For EDS analysis, the characteristic X-rays emitted from the sample due to the interaction with the electron beam are collected. Thus, we can analyse the elemental composition of the investigated materials with a high spatial resolution. In EELS we record the energy transferred from the beam electrons to the sample. This can be used to investigate the local electronic structure of the particles/material.
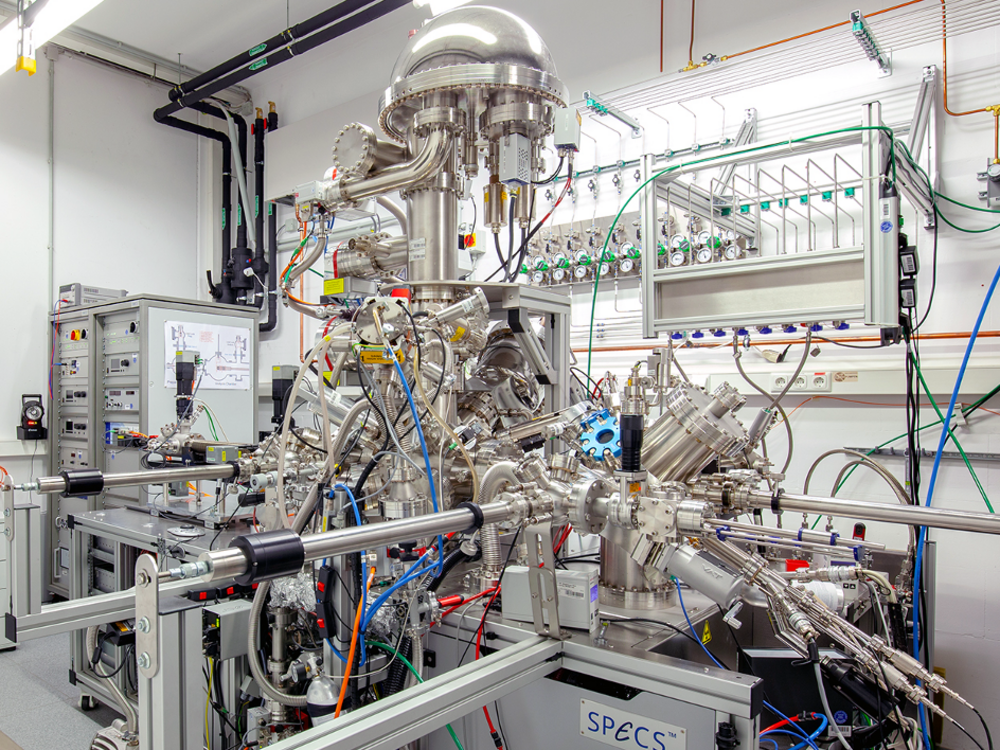
Using the second focus of our group, X-ray photoelectron spectroscopy (XPS), we can determine the chemical composition and the electronic structure of the surface of solids (Fig. 1b). Using X-ray radiation, photoelectrons and Auger-electrons are released from the sample, and their energy is determined, which yields information about the surface of the investigated samples.
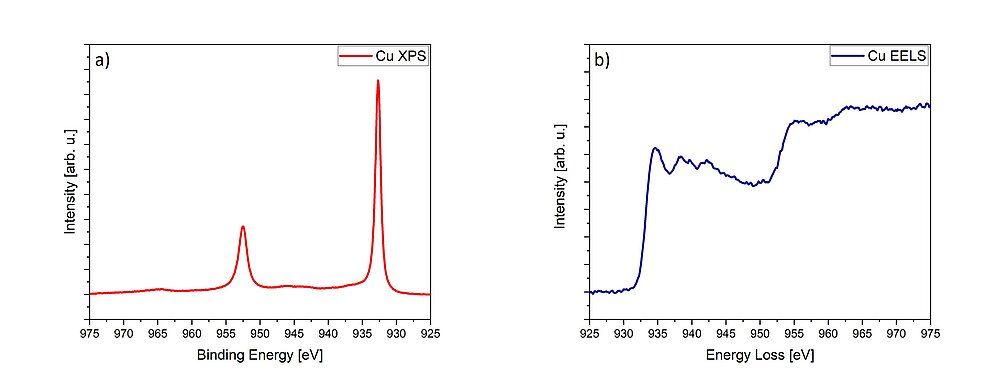
EELS and XPS are related, since they both give information about the local electronic structure, oxidation state and chemical bonding of the investigated materials.1,2 While information about the occupied density of states is collected using XPS (Fig. 2a), EELS accesses the unoccupied density of states (Fig. 2b). Both spectroscopic methods can additionally probe the valence- and conduction band structure.
Life cycle of TEM- and XPS data
Analytical electron microscopy and XPS are important and widely used parts of the characterisation routines in modern catalysis research institutes. Here, a careful and standardised design of the experiments and the choice of correct analytical methods are of greatest importance. The research efficiency can be greatly improved if researchers in the laboratories can perform standardised investigations, such as material screening or qualitative analysis of material composition, themselves. In our group, we apply the standardised procedures and workflows developed within the ChemiTEM project at the Fritz-Haber-Institute of the Max-Planck-Society in collaboration with Thermo Fisher Scientific.3 Furthermore, we expand these procedures on the spectroscopic techniques available in our group.
The next step in the life of TEM and XPS data is the detailed analysis. Only this ensures that the information gained can be related to data from other experiments, yielding knowledge about the properties of new model catalysts. For this, the recorded data need to be easily searched, analysed and reused. To achieve this goal, our group is also integrated into the FAIRmat consortium.4
Method development
A special focus of our group’s research is the investigation of inertly transferred samples (i.e., samples put into the equipment without contact to air). This is essential, since contact to air would change the investigated catalysts. To achieve this, unique self-developed methods are available to us, facilitating optimal workflows during TEM- and XPS measurements.
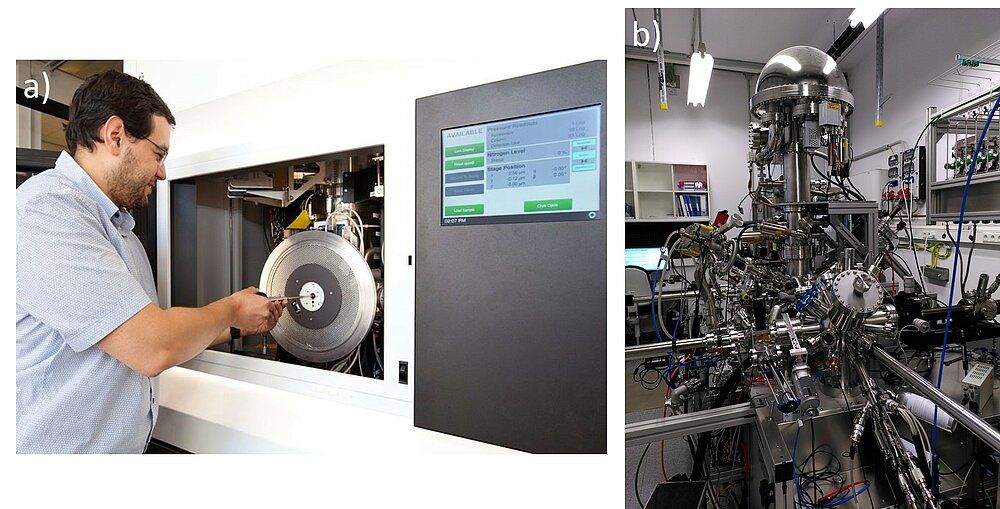
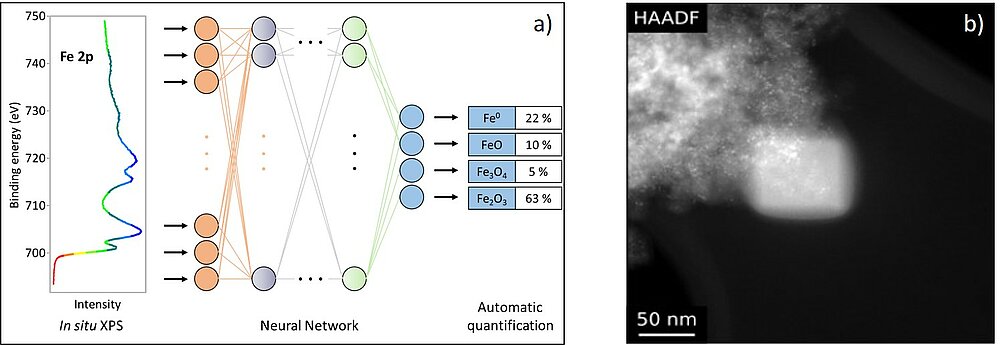
The execution of spectroscopic experiments can be very challenging and complex, as can the evaluation of the results. Therefore, comparison with reference spectra and simulations are necessary for an in-depth analysis. However, it becomes increasingly difficult to analyse sets of data manually. We perform EELS simulations using a combination of software packages based on density functional theory (DFT) and the Bloch wave formalism.5,6 For the evaluation of XPS data we use and develop many modern methods of data science and machine learning, including Monte-Carlo-simulations, unsupervised machine learning and neural networks. A current project focuses on the use of convolutional neural networks for the automated analysis of complex XP-spectra of transition metals (see Fig. 3a).
A challenge for TEM investigations is the carbon contamination of the sample surface, which impairs the image quality and the spectroscopic analyses (Fig. 3b).7 Our current research involves the chemical analysis of the contamination products and aims at developing individualised methods to avoid contamination tailored to each sample.
Research topics
We support all research groups of the institute with our electron microscopical and surface characterisation methods. Wherever analytical characterisation on smallest length scales is required, our equipment is applied. In cooperation with the analytical methods employed by other groups, our methods are used for the current projects at the institutes.
Within a further project, which is part of the UniSysCat cluster of excellence, we investigate a series of bimetallic nanocatalysts in order to understand the effect of particle morphology (size, composition, arrangement, etc.) on selectivity and conversion in specific reactions.8 In this way, differences in the catalytic performance resulting from changes to the synthesis and pretreatment of the catalysts can be related to changes in the geometric and electronic structure, as studied in the TEM.
Equipment
Thermo Scientific Talos F200X G2
- X-FEG field emission gun
- Acceleration voltage: 80 kV and 200 kV
- TEM information limit: 0.12 nm
- STEM resolution: 0.16 nm
- Ceta 16M camera
- BF, DF, HAADF, iDPC Detectors
- SuperX EDX system
- Solid angle: 0.9 sr
- Energy resolution: 136 eV
- Gatan Continuum S
- Energy resolution: 0.8 eV
- TEM & STEM analytical tomography
Jeol JEM-ARM200F
Jointly operated with the Fritz Haber Institute of the Max Planck Society
- Cold field emission gun
- Acceleration voltage: 80 kV and 200 kV
- Cs-corrector for objective and condenser lens system
- TEM information limit: 0.10 nm
- STEM resolution: 0.08 nm
- Gatan Orius SC200D camera
- Gatan OneView IS camera
- BF, DF, HAADF, SE detectors
- JEOL EDX system
- Solid angle: 0.8 sr
- Energy resolution: 133 eV
- Gatan GIF Quantum ER
- Energy resolution: 0.3 eV
- BF, DF, HAADF detector system
NAP-XPS
- Specs PHOIBOS 150 NAP 1D-DLD hemispherical energy analyzer
- Acceptance angle: +/- 22°
- 1D-DLD detector
- Energy resolution: < 30 meV
- Aperture differential pumping stage:
300 µm (> 5 mbar), 800µm (< 5 mbar)
- Specs µFOCUS 500
- Monochromatic Al Kα source: hν = 1486.6 eV
- Coherent Compact Evolution IR Diode LASER
- Specs UVS 300 NAP UV light source
- Specs EQ 22/35 electron source
- mks e-Vision 2 mass spectrometer
- Preparation chamber
- Specs IQE 11/35 ion source
- Specs EBE-4 electron beam evaporator
References
[1] Egerton, Electron Energy-Loss Spectroscopy in the Electron Microscope, Springer, 2011
[2] Surface Analysis, Briggs and Grant, IM Publications LLP, 2003
[3] Hetaba et al., Chemistry-Methods 2021, 1, 401-407
[4] https://www.fairmat-nfdi.eu/fairmat/consortium
[5] Hetaba et al., PRB 85, 205108 (2012)
[6] Hetaba et al., Micron 63 (2014), 15-19
[7] McGilvery et al., Micron 43 (2012), 450-455