Dr. Sergio A. V. Jannuzzi - Spectroscopy and Reactivity of Molecular Catalysts
- Dr. Sergio Augusto Venturinelli Jannuzzi
- Project leader
- Spectroscopy and Reactivity of Molecular Catalysts
- Inorganic Spectroscopy
- +49 (0)208 306 - 3586
- sergio.jannuzzi(at)cec.mpg.de
- Room: 506
Vita
Awards
- Marie Skłodowska-Curie Actions Seal of Excellence, European Research Commission (2018)
- Lindau alumnus of the 67th Lindau Nobel Laureate Meeting, Lindau Nobel Laureate Meeting Council (2017)
- Selected participant of the Baden-Württemberg Post Conference Programme of the Lindau Nobel Laureate Meeting, Baden-Württemberg International (2017)
- NanoSystem Initiative Munich Research Award, LMU Munich (2010)
- Lavoisier Award of Best Student in Chemistry 2005-2009, Regional Council of Chemistry, 4th Region, Brazil (2009)
- II Assintecal/Petrobras Award in Adhesive Technology and Shoe Fastening Processes, Brazilian Association of Companies of Components for Leather, Footwear and Devices (2007)
Publications
Publications
Full publications list | ORCID
Selected MPI CEC publications
- Keilwerth, M., Mao, W., Venturinelli Jannuzzi, S. A., Grunwald, L., Heinemann, F. W., Scheurer, A., et al. (2023). From Divalent to Pentavalent Iron Imido Complexes and an Fe(V) Nitride via N-C Bond Cleavage. Journal of the American Chemical Society, (145), 873-887. doi:10.1021/jacs.2c09072.
- W. Mao, D. Fehn, F.W. Heinemann, A. Scheurer, M. van Gastel, S.A.Venturinelli Jannuzzi, S. DeBeer, D. Munz and K. Meyer. “Umpolung in a Pair of Cobalt(III) Terminal Imido/Imidyl Complexes”. Angewandte Chemie International Edition (2022) 61, e202206848. https://doi.org/10.1002/anie.202206848
- C Souilah, SAV Jannuzzi, D Demirbas, S Ivlev, M Swart, S DeBeer and A Casitas. “Synthesis of Fe(III) and Fe(IV) Cyanide Complexes Using Hypervalent Iodine Reagents as Cyano-Transfer One-Electron Oxidants”. Angewandte Chemie International Edition (2022), 61.22, e202201699. https://doi.org/10.1002/anie.202201699
- I Gerz, SAV Jannuzzi, KT Hylland, C Negri, DS Wragg, S Øien-Ødegaard, M Tilset, U Olsbye, S DeBeer and M Amedjkouh. “Structural Elucidation, Aggregation and Dynamic Behaviour of N,N,N,N-Copper(I) Schiff Base Complexes in Solid and in Solution: a Combined NMR, X-ray Spectroscopic and Crystallographic Investigation”. European Journal of Inorganic Chemistry (2021), 46, 4762–4775. https://doi.org/10.1002/ejic.20210072
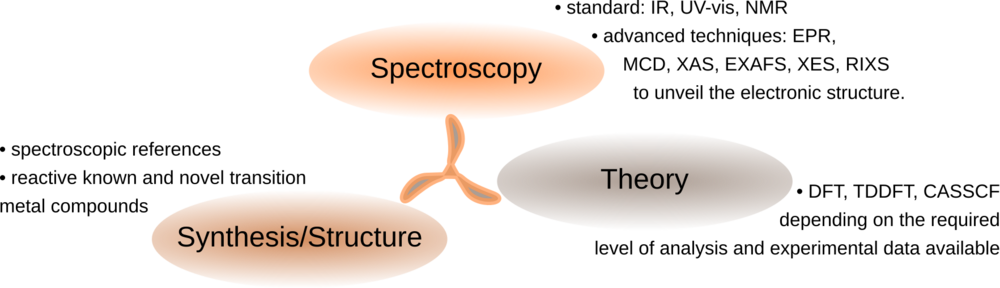
Spectroscopy and Reactivity of Molecular Catalysts

Numerous natural and man-designed transformations of fundamental importance to our daily lives are enabled by transition metal molecular catalysts in solution. Spectroscopy overall provides the means to interrogate not only reactants and products, but also the nature of key reactive species responsible for the desired reactivity. The spectroscopic observables are often not clearly mapped to the atomic structural domain as a consequence of intricate quantum mechanical selection rules of light-matter interaction. To close the loop, molecular modeling is essential to provide chemical insights and to propose electronic structural descriptions consistent with experimental data.
In our group, we employ the synthesis-spectroscopy-theory tripod to drive fundamental research on the key characteristics that enable first-row transition metals to perform C-H functionalization.
While enzymes serve as inspiration to design and improve synthetic catalysts, the investigation of molecular models shed light on enzyme’s structure and mechanism. Lytic polysaccharide monooxygenase1 (LPMO) has a solvent-exposed monocopper site able to activate C-H bonds of insoluble polysaccharide substrates, thus breaking them into smaller segments with potential use as renewable feedstock in fermentative processes. The natural oxygen atom source – O2 or H2O2 – is currently debated.2 The mechanism as well as the key reactive intermediate is unknown. The importance of the first coordination sphere relative to distal amino-acid side chains in the second coordination sphere is also of extreme interest to the design of artificial catalytic systems and confined spaces.
References
1. Vaaje-Kolstad, G.; Westereng, B.; Horn, S.J.; Liu, Z.; Zhai, H.; Sørlie, M.; Eijsink, V.G.; An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 2010, 330(6001), 219-22.
2. Bissaro, B.; Røhr, Å.K.; Müller, G.; Chylenski, P.; Skaugen, M.; Forsberg, Z.; Horn, S.J.; Vaaje-Kolstad, G.; Eijsink, V.G.H.; Oxidative cleavage of polysaccharides by monocopper enzymes depends on H2O2. Nat Chem Biol. 2017, 13(10):1123-1128.