Prof. Dr. Regina Palkovits - Solid Molecular Catalysts
- Prof. Dr. Regina Palkovits
- Max Planck Fellow
- Solid Molecular Catalysts
- +49 (0)208 306 - (0)241 802 649
- Palkovits(at)itmc.rwth-aachen.de
- Room: 108
Vita
| Diploma of Chemical Engineering | Technical University Dortmund/ Germany (1998-2003) |
| PhD Student | Max-Planck-Institut für Kohlenforschung, Mülheim a.d. Ruhr/ Germany (2003-2006) |
| PostDoc | Utrecht University/ The Netherlands (2007) |
| Group Leader | Max-Planck-Institut für Kohlenforschung, Mülheim/ Germany (2008-2010) |
| Associate Professor | Nanostructured Catalysts, ITMC, RWTH Aachen University/ Germany (2010-2013) |
| Full Professor | Heterogeneous Catalysis & Chemical Technology, ITMC, RWTH Aachen University/ Germany (since 2013) |
| Acting Director | Institute of Chemical Technology & Makromoleculare Chemistry (ITMC), RWTH Aachen University/ Germany (since 2015) |
| Max Planck Fellow | MPI CEC (since 2019) |
Fellowships & Awards
- 2019 Max Planck Fellow at the Max-Planck Institute of chemical Energy Conversion
- 2019 EFCATS Young Researcher Award
- 2019 Exxon Mobil Science & Engineering Award
- 2017 DECHEMA Award for outstanding scientific contributions, Dechema/Germany
- 2015 FAMOS for family (award for family-friendliness of RWTH Aachen University
- 2013 Max-Buchner Research Fellowship
- 2011 Selected for the Capital-Project Young Elite “Four time forty below forty”
- 2011 Award „100 Women of tomorrow“ of the initiative „Germany – Country of Ideas“
- 2010 Innovation Award of North-Rhine-Westphalian Academy of Science/ Germany
- 2010 Robert Bosch Junior Professorship of Robert Bosch Foundation/ Germany
- 2010 Jochen-Block Award of the German Catalysis Society/ Germany
- 2009 Award for “Comprehensible Science” of GKSS, Helmholtz Society/ Germany
- 2008 „Fast-Track Fellowship” of Robert Bosch Foundation/ Germany
- 2006 Hendrik Casimir – Karl Ziegler Research Award of Royal Netherlands Academy of Arts and Science and North Rhine-Westphalia Academy of Science and Arts
Publikationen
Publications
Full publications list | ORCID: 0000-0002-4970-2957
- Kaya, K., Ditz, D., Jaworski, A., Chen, J., Monti, S., Barcaro, G., Budnyk, S., Slabon, A., Palkovits, R. (2023). Enhanced Solar CO2 Photoreduction to Formic Acid by Platinum Immobilization on Bipyridine Covalent Triazine Framework with Defects. Advanced Sustainable Systems, (xx): 2300071, pp. 1-12. doi:10.1002/adsu.202300071.
- Favaro, M. A., Yang, J., Ditz, D., Kucukkececi, H., Alkhurisi, M. H., Bergwinkl, S., Thomas, A., Quadrelli, E. A., Palkovits, R., Canivet, J., Wisser, F. M. (2023). Pyrene- and Bipyridine-based Covalent Triazine Framework as Versatile Platform for Photocatalytic Solar Fuels Production. ChemCatChem, (15): e202300194, pp. 1-7. doi:10.1002/cctc.202300197.
- Sackers, N. M., Iemhoff, A., Sautet, P., Palkovits, R. (2023). Understanding the structure of isolated iridium sites anchored on a covalent triazine framework. Catalysis Science & Technology, (13),2652-2655 doi:10.1039/d3cy00232b.
- Mürtz, S. D., Musialek, F., Pfänder, N., Palkovits, R. (2023). Bimetallic PtCu/C Catalysts for Glycerol Assisted Hydrogen Evolution in Acidic Media. ChemElectroChem, (10): e202201114, pp. 1-7. doi:10.1002/celc.202201114.
- Vennewald, M., Sackers, N. M., Iemhoff, A., Kappel, I., Weidenthaler, C., Meise, A., Heggen, M., Dunin-Borkowski, R. E., Keenan, L., Palkovits, R. (2023). Dynamics of palladium single-atoms on graphitic carbon nitride during ethylene hydrogenation. Journal of Catalysis, (421), 134-144. doi:10.1016/j.jcat.2023.03.011.
- Li, Z., Yi, X., Wang, Q., Li, Y., Li, D., Palkovits, R., Beine, K., Liu, C., Wang, X. (2023). Selective Production of Glycolic Acid from Cellulose Promoted by Acidic/Redox Polyoxometalates via Oxidative Hydrolysis. ACS Catalysis, (13), 4575-4586. doi:10.1021/acscatal.2c05568.
- Yan, D., Mebrahtu, C., Wang, S., Palkovits, R. (2023). Innovative Electrochemical Strategies for Hydrogen Production: From Electricity Input to Electricity Output. Angewandte Chemie, International Edition in English, (62) e202214333, pp. 2-21. doi:10.1002/anie.202214333.
- Sebastian, J., Mebrahtu, C., Palkovits, R. (2023). Influence and stability of the surface density of MoOx on TiO2 in deoxydehydration: structure-activity correlations. Catalysis Science & Technology, (13), 1087-1097. doi:10.1039/d2cy01854c.
- Iemhoff, A., Vennewald, M., Palkovits, R. (2023). Single-Atom Catalysts on Covalent Triazine Frameworks: at the Crossroad between Homogeneous and Heterogeneous Catalysis. Angewandte Chemie, International Edition in English, (62): e202212015, pp. 1-15. doi:10.1002/anie.202212015.
- Merchan, A. L., Fischöder, T., Hee, J., Lehnertz, M. S., Osterthun, O., Pielsticker, S., Schleier, J., Tiso, T., Blank, L. M., Klankermayer, J., Kneer, R., Quicker, P., Walther, G., Palkovits, R. (2022). Chemical recycling of bioplastics: technical opportunities to preserve chemical functionality as path towards a circular economy. Green Chemistry, (24), 9428-9449. doi:10.1039/d2gc02244c.
- Kipshagen, A., Baums, J. C., Hartmann, H., Besmehn, A., Hausoul, P. J. C., Palkovits, R. (2022). Formic acid as H-2 storage system: hydrogenation of CO2 and decomposition of formic acid by solid molecular phosphine catalysts. Catalysis Science & Technology, (12),5649-5656. doi:10.1039/d2cy00608a.
Top 10 Publications
- C. Broicher, M. Klingenhof, M. Frisch, S. Dresp, N. M. Kubo, J. Artz, J. Radnik, S. Palkovits, A. K. Beine, P. Strasser, R. Palkovits, Catalysis Science & Technology 2021, doi: 10.1039/d1cy00905b.
- M. O. Haus, A. Meledin, S. Leiting, Y. Louven, N. C. Roubicek, S. Moos, C. Weidenthaler, T. E. Weirich, R. Palkovits, ACS Catalysis 2021, 11, 5119-5134.
- C. Broicher, F. Zeng, N. Pfänder, M. Frisch, T. Bisswanger, J. Radnik, J. M. Stockmann, S. Palkovits, A. K. Beine, R. Palkovits, ChemCatChem 2020, 12, 5378-5384.
- X. Wang, A. K. Beine, P. J. C. Hausoul, R. Palkovits, ChemSusChem 2020, 13, 126-130.
- X. Wang, A. K. Beine, P. J. C. Hausoul, R. Palkovits, ChemCatChem 2019, 11, 4123-4129.
- R. Palkovits, S. Palkovits, ACS Catalysis 2019, 9, 8383-8387.
- A. K. Beine, C. Broicher, Q. Hu, L. Mayerl, T. Bisswanger, H. Hartmann, A. Besmehn, S. Palkovits, A.-H. Lu, R. Palkovits, Catalysis Science & Technology 2018, 8, 6311-6315.
- A. K. Beine, A. J. D. Krüger, J. Artz, C. Weidenthaler, C. Glotzbach, P. J. C. Hausoul, R. Palkovits, Green Chemistry 2018, 20, 1316-1322.
- P. J. C. Hausoul, A. K. Beine, L. Neghadar, R. Palkovits, Catalysis Science & Technology 2017, 7, 56-63.
- I. Delidovich, P. J. C. Hausoul, R. Pfützenreuter, M. Rose, R. Palkovits, Chemical Reviews 2016, 116, 1540-1599.
Open positions
The group "Solid Molecular Catalysts" is always looking for new talented students. Feel free to contact Dr. Anna Katharina Beine for this purpose.
Inquiries should include a motivation letter as well as a CV and a grade summary.
Specific projects for which we are currently looking for collaborators are listed below:
- Zeolites as solid catalysts for bio-based tandem reactions (research work / bachelor thesis)
- Hydroformylation of hexene with modified heteropolyacids (research work / bachelor thesis).
- Investigation of the reaction network for the hydrogenolysis of 1,4-anhydroxylitol (research work / bachelor thesis)
- Catalytic production of diformylxylose as fuel additive (research work / bachelor thesis)
- Investigation of the electrochemical conversion of xylose and furfural (research work / master thesis)
- Development of molecular Ni catalysts for the conversion of CO and H2 to formaldehyde (research work / master thesis)
- Ru nanoparticles on nitrogen-containing carbons for the hydrogenolysis of xylitol (research work / master thesis)
- SBA-15 functionalization for the immobilization of heteropolyacids as catalysts in biorefinery (master thesis)
- Investigation of the continuous conversion of hemicellulose using heteropolyacid on carbon supports (master thesis with the option to continue as a PhD student)
Research - Solid Molecular Catalysts
With heterogeneous catalysis and materials development as our core competence, we address global challenges by developing sustainable chemical reactions and processes. Our research focuses on the development of materials for the chemical conversion of renewable energy and carbon sources. This includes, for example, electrochemical water splitting, but also the conversion of renewable raw materials such as biomass. We are particularly interested in understanding the mechanisms and compositions of the developed catalysts in order to further improve them or use them in other reactions. In this way, we are contributing to shaping a greener future.[1]
Our current research focuses are:
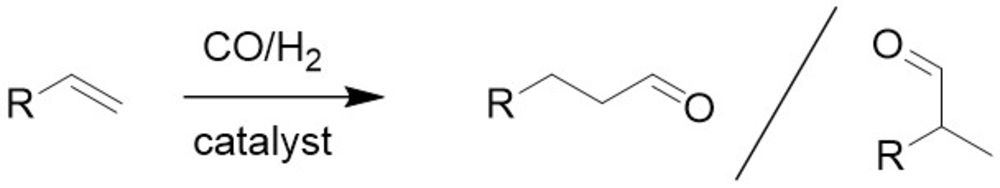
Hydroformylation in the liquid phase
Hydroformylation remains one of the largest homogeneously catalyzed reactions in industrial chemistry. Together with our project partners at MPI-CEC and Hamburg University, we are developing new homogeneous Co-based catalysts to successfully catalyze the reaction. In addition to the optimization of the process parameters, the immobilization of the catalysts on suitable heterogeneous support materials is being investigated.
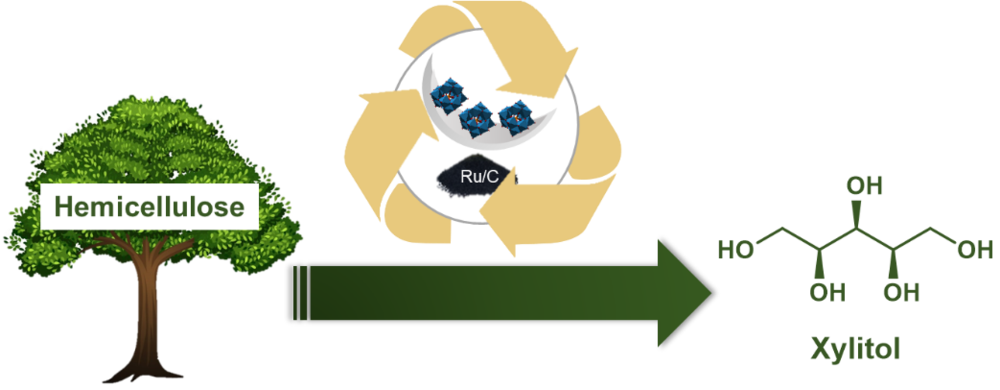
Immobilized heteropolyacids as catalysts for the hydrolytic hydrogenation of hemicellulose to xylitol
In times of climate change and diminishing fossil resources, the transition to renewable raw materials such as lignocellulose is essential. In this project, the hydrolytic hydrogenation of hemicellulose to xylitol is investigated. Xylitol is both a widely used sugar substitute and an important platform chemical for biorefinery. In a tandem reaction, the polysaccharide is first depolymerized to xylose by acid-catalyzed hydrolysis, followed by metal-catalyzed hydrogenation to xylitol. In this context, Brønsted acidic heteropolyacids (HPAs) in combination with Ru/C were shown to be an efficient catalyst system. Although the HPAs exhibit excellent catalytic activity, their good solubility in water and various organic solvents makes their recycling difficult. To address this challenge, the immobilization of HPAs on heterogeneous supports is investigated. The solid acid catalysts prepared are characterized in depth and tested in the reaction. The construction of a suitable reactor is planned for the investigation of long-term stability.
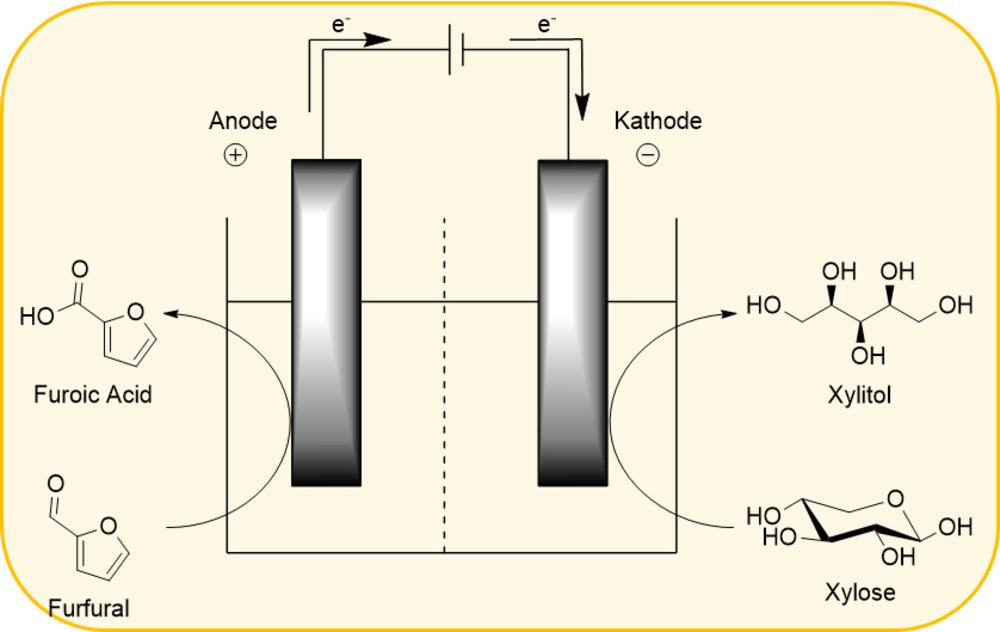
Electrode development for electrochemical applications
The oxygen evolution at the anode of the electrochemical water splitting reaction has already been extensively studied in the group.[2-4] Advanced studies are planned for the future.
In addition, the electrochemical conversion of biobased molecules is in the focus of research. Here, conversions along the value chain of hemicellulose are to be investigated in particular in 200% cells. HPAs are used as catalysts, either as electrolytes or immobilized onto the conducting electrode.
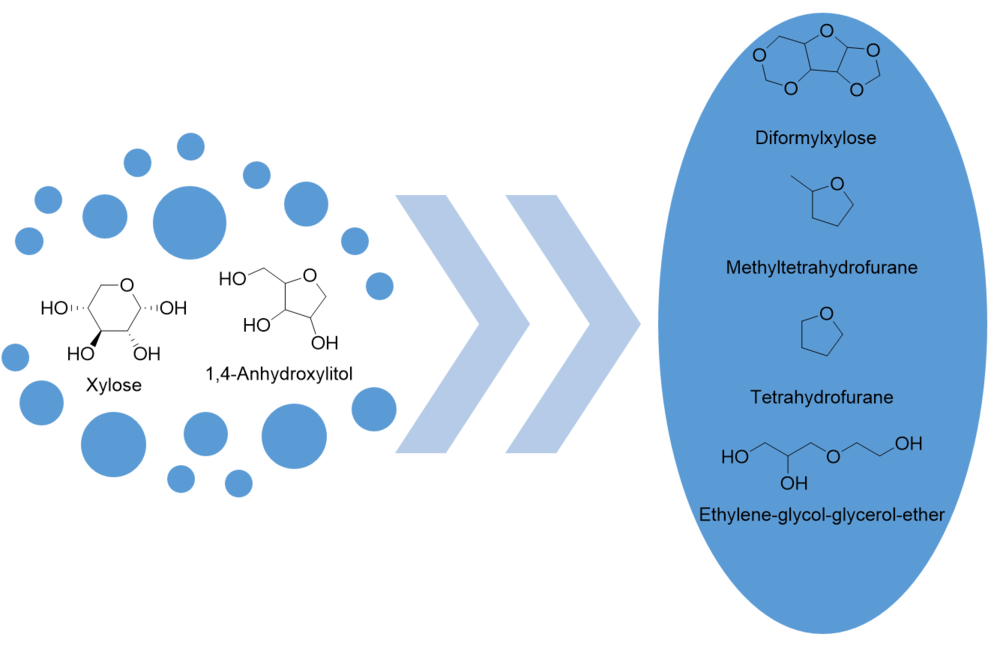
New reaction pathways for biorefinery
In addition to the already known biorefinery conversions and products, other new reaction pathways are opening up, leading to products that can be used, for example, in the polymer industry, as solvents or fuel additives. Thus, starting from substrates such as xylose or 1,4-anhydroxylitol, access to difromylxylose[5], tetrahydrofuran or methyltetrahydrofuran and ethylene-glycol-glycerol-ether is made possible.
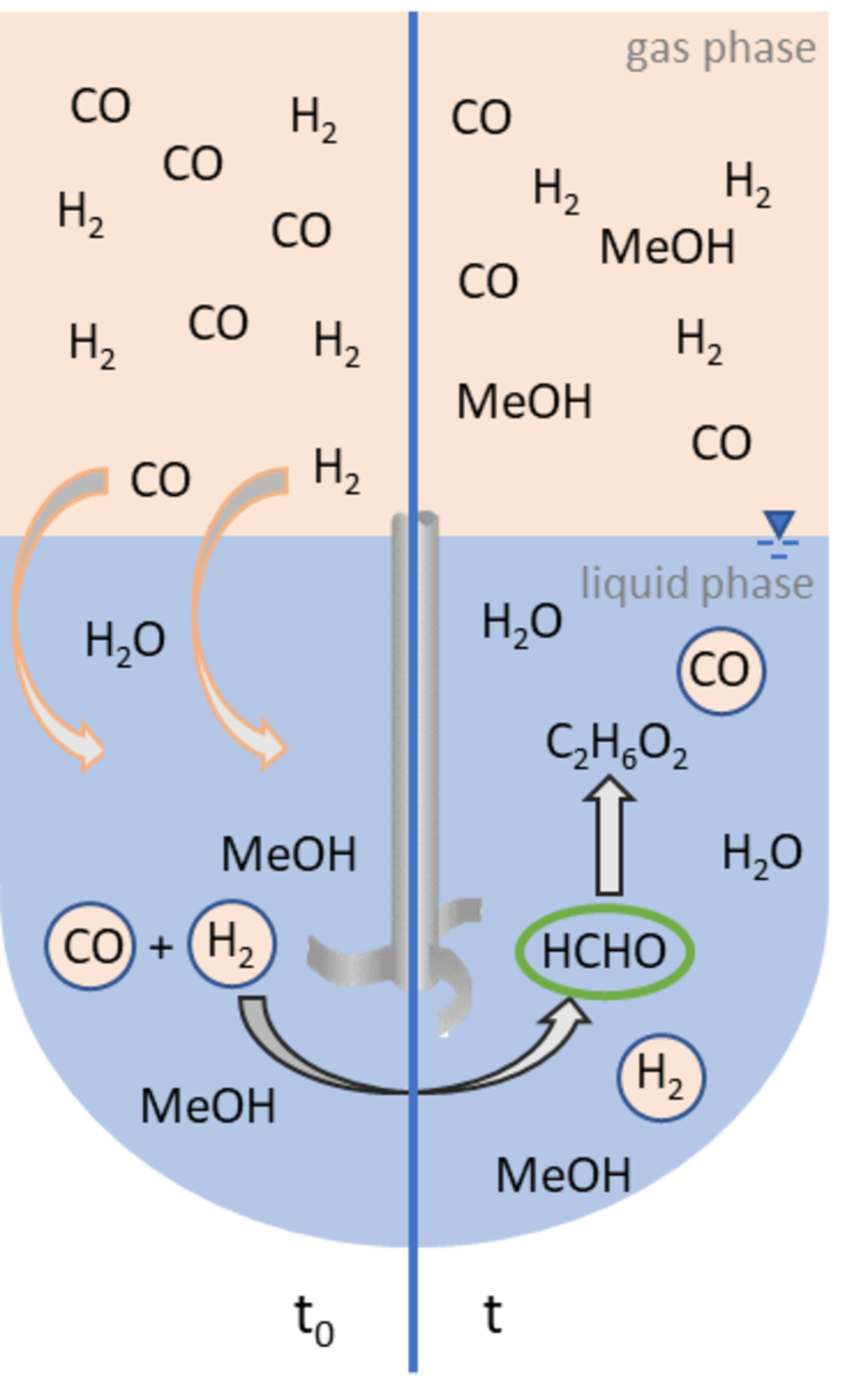
Conversion of H2, CO and CO2 to high value products
Formaldehyde is a bulk chemical and serves as substrate for the production of products such as polymers and paints. It is also an important substrate for the production of oxymethylene ethers, which can be used as sustainable fuels and fuel additives or as solvents. The main focus of our research is the conversion of CO and later CO2 with H2 to formaldehyde. The reaction shall be realized in aqueous solution with the help of solid molecular catalysts. Intelligent catalyst design and a profound understanding of the reaction will allow the development of new reaction pathways.
References
| [1] | A. K. Beine, ChemCatChem 2021, 13, 532-533. |
| [2] | A. K. Beine, C. Broicher, Q. Hu, L. Mayerl, T. Bisswanger, H. Hartmann, A. Besmehn, S. Palkovits, A.-H. Lu, R. Palkovits, Catalysis Science & Technology 2018, 8, 6311-6315. |
| [3] | C. Broicher, F. Zeng, N. Pfänder, M. Frisch, T. Bisswanger, J. Radnik, J. M. Stockmann, S. Palkovits, A. K. Beine, R. Palkovits, ChemCatChem 2020, 12, 5378-5384. |
| [4] | C. Broicher, M. Klingenhof, M. Frisch, S. Dresp, N. M. Kubo, J. Artz, J. Radnik, S. Palkovits, A. K. Beine, P. Strasser, R. Palkovits, Catalysis Science & Technology 2021, doi: 10.1039/d1cy00905b. |
| [5] | A. O. Komarova, G. R. Dick, J. S. Luterbacher, Green Chemistry 2021, 23, 4790-4799. |
| [6] | A. M. Bahmanpour, A. Hoadley, A. Tanksale, Green Chemistry 2015, 17, 3500-3507. |