Dr. Gregor Kemper - Organometallic CO2 Chemistry
- Dr. Gregor Kemper
- Group leader
- Organometallic CO2 Chemistry
- Molecular Catalysis
- +49 (0)208 306 - 3172
- gregor.kemper(at)cec.mpg.de
Vita
| B.Sc. | University of Konstanz (2008-2011) |
| M.Sc. | University of Konstanz - Prof. Dr. S. Mecking (2012-2015) |
| Dr. rer. nat. | RWTH Aachen University, Institute for Technical and Macromolecular Chemistry - Prof. Dr. Walter Leitner (2016-2020) |
| PostDoc | RWTH Aachen University, Institute for Technical and Macromolecular Chemistry - Prof. Dr. Walter Leitner (2021-2022) MPI CEC, Molecular Catalysis - Prof. Dr. Walter Leitner (2023) |
| Group leader | 'Organometallic CO2 Chemistry', MPI CEC (seit 2024) |
Publications
Full publications list | ORCID
Selected MPI CEC publications
- Voit, G.; Jenthra, S.; Hölscher, M.; Weyhermüller, T.; Leitner, W. Reversible insertion of carbon dioxide at phosphine sulfonamido PdII–aryl complexes. Organometallics 2020, 39 (24), 4465-4473.
- Kemper, G.; Hölscher, M.; Leitner, W. Pd (II)-catalyzed carboxylation of aromatic C─ H bonds with CO2. Science Advances 2023, 9 (5), eadf2966.
- Singh, A.; Kemper, G.; Weyhermueller, T.; Kaeffer, N.; Leitner, W. Activated Mn‐MACHO Complexes Form Stable CO2 Adducts. Chemistry–A European Journal 2023, e202303438.
Group members
Open positions
The Organometallic CO2 Chemistry group is always looking for interested and talented students, PhD students and PostDocs. Candidates who are enthusiastic about organometallic chemistry, would like to understand reaction mechanisms and to conduct fundamental research in a motivating environment that might contribute to applications for a more sustainable world are cordially invited to contact Gregor Gregor Kemper.
Research in the Group Organometallic CO2 Chemistry
In the "Organometallic CO2 Chemistry" team, we are working on developing homogeneous transition metal catalysts that can be used to utilize carbon dioxide (CO2) in novel reactions. In combination with other non-fossil raw materials, this greenhouse gas can thus be converted into fine and base chemicals as well as energy storage media.
We are pursuing this research goal because, in view of the climate crisis and the continuing release of toxic and persistent chemicals into the environment, a fundamental change is needed in the chemical industry and the energy sector. While the latter can - at least theoretically - be completely decarbonized, the products required for chemical synthesis will continue to be largely based on the element carbon. A circular and holistic chemical industry must therefore be established in which the carbon contained in the products is reused in synthesis at the end of their life. In addition, in the search for more efficient products or for alternative reaction pathways of established products, aspects such as the avoidance of toxic or persistent waste materials, the increase in atom economy of chemical reactions and the avoidance of toxic, fossil and conflict-causing raw materials must be considered from the outset and sustainability must be understood as an integral part of product performance1, 2.
In addition to the use of suitable renewable organic compounds and hydrogen produced from renewable energies, the conversion of the greenhouse gas CO2 has the potential to make a major contribution to this defossilization of the chemical value chain. It is a non-toxic, non-flammable and cheap source of carbon that is (more than enough) present in our atmosphere and continuously emitted on a very large scale. However, due to its properties as a very stable and inert molecule, the use of suitable catalysts is necessary for its conversion. To this end, our group is developing homogeneous transition metal complexes that enable chemical transformations with high selectivity even under mild reaction conditions. In addition, these compounds have outstanding accessibility for analytical methods, so that the underlying reaction mechanisms can be understood and influenced very well from the outset. This opens up the possibility of rationally developing tailor-made catalysts to enable the selective and atom efficient conversion of available starting materials under desired reaction conditions to the required products. In line with Planck's motto: "Insight must precede application."
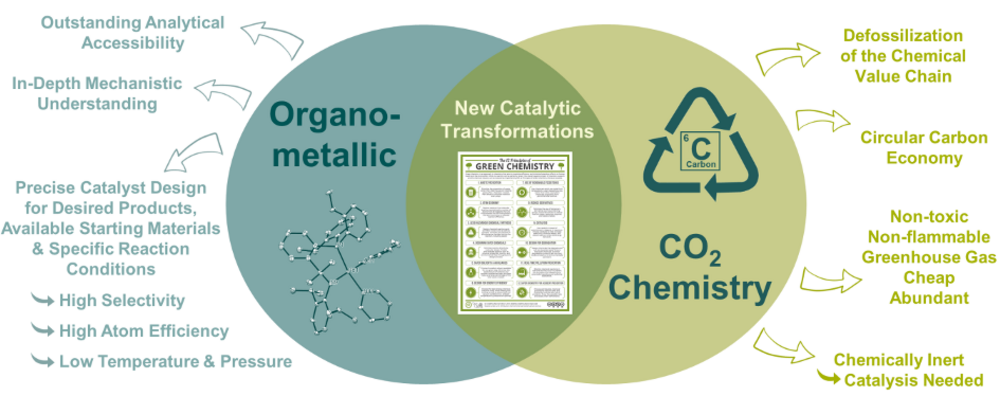
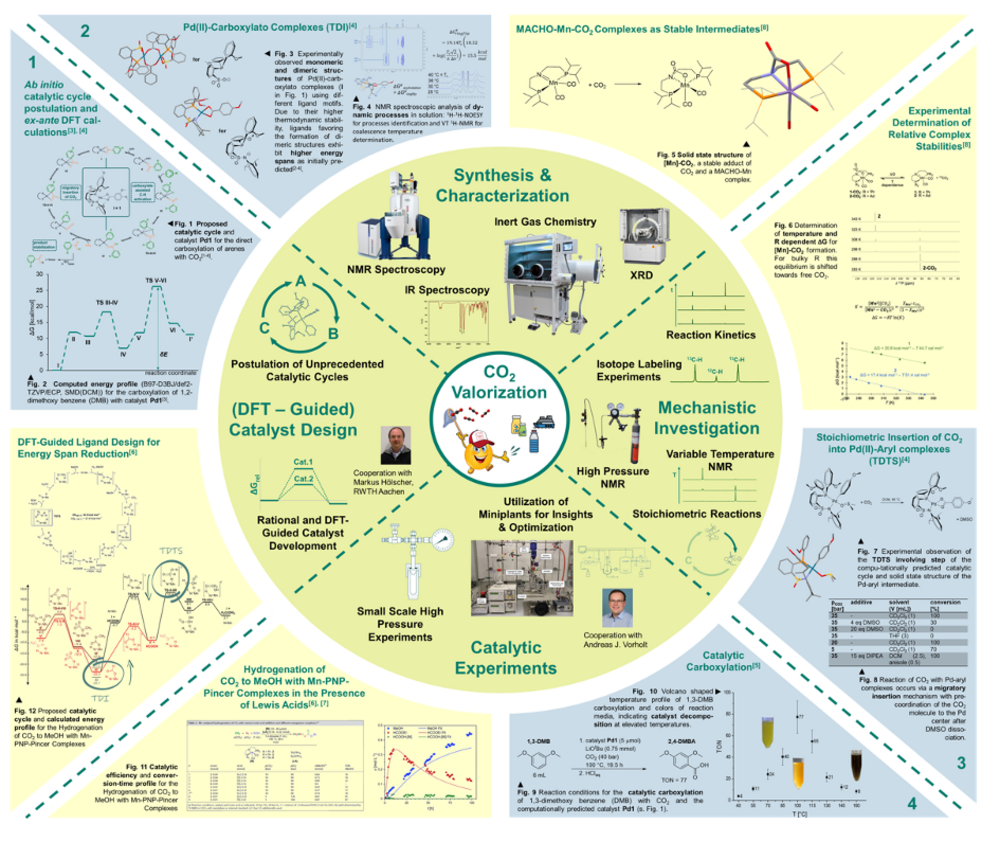
Part of our research activity therefore consists of synthesis under inert gas conditions and analysis of the resulting compounds using spectroscopic and X-ray diffractometric methods. The resulting compounds are used in catalysis experiments of various scale with the aim of developing applicable reaction systems. In addition, we pay great attention to the investigation of the underlying reaction mechanisms, with NMR spectroscopic studies taking center stage. This mechanistic understanding is supported by a close collaboration with the group "Computational Methods in Catalysis" led by Dr. Markus Hölscher at RWTH Aachen University, with whose help we not only use computational methods (DFT) to explain experimental observations, but also want to extend them to the prediction of catalyst structures and hitherto unknown catalytic cycles (Fig. 4 inside).
Carboxylation of simple arenes
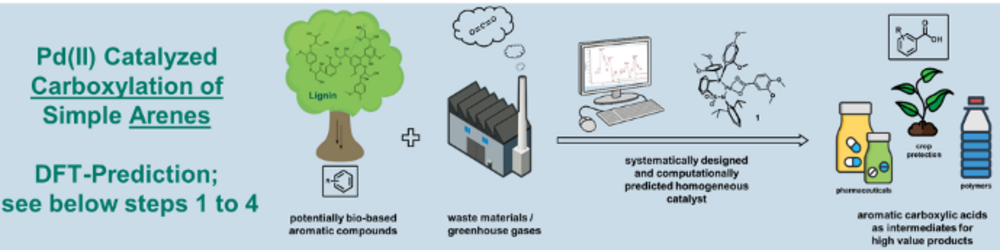
One example of this research approach is the homogeneously catalyzed carboxylation of aromatic C-H bonds with CO2 (Fig. 2). This is an atom efficient catalytic process for the synthesis of aromatic carboxylic acids, which are a common structural motif in polymers, fine chemicals and biologically active compounds. To realize this reaction, a novel catalytic cycle was postulated, for which possible catalysts were proposed ex-ante via computational chemistry calculations3. The catalytic activity of phosphine sulfonamido-Pd(II) complexes was subsequently confirmed experimentally4, 5. We are currently working on making this system more effective using new catalysts and on making a broader spectrum of arenes from renewable sources accessible for this conversion by mechanism-based optimization of the reaction conditions.
Hydrogenation of CO2 to methanol with 3d metals
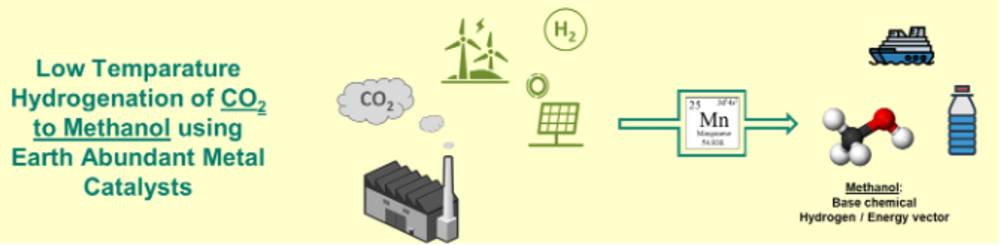
We are also conducting research into the catalytic hydrogenation of CO2 (Fig. 3). Methanol can be regarded as a reaction product of particular interest. This alcohol is not only of central importance as a base chemical (annual production volume >100 Gt), whose broad application potential can be further increased by ongoing research in this field, but can also be used as an energy storage medium in the future.
We are interested in finding organometallic catalyst systems that enable methanol synthesis from CO2 and elemental hydrogen under mild reaction conditions and with high selectivity. In particular, we are investigating complex compounds that dispense with precious metals and instead use 3d metals, such as manganese, as the central atom, which are earth abundant and can therefore be obtained more cheaply and with lower greenhouse gas emissions. Based on initial successful experiments on the synthesis of methanol with manganese-MACHO complexes6, 7, we are currently working on improving this system through targeted mechanism studies8 and catalyst development - partly supported by computational chemistry - to the point where this important building block of a circular carbon economy can be produced economically.
(1) Zimmerman, J. B.; Anastas, P. T.; Erythropel, H. C.; Leitner, W. Designing for a green chemistry future. Science 2020, 367 (6476), 397-400.
(2) Artz, J.; Müller, T. E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable conversion of carbon dioxide: an integrated review of catalysis and life cycle assessment. Chemical reviews 2018, 118 (2), 434-504.
(3) Hölscher, M.; Kemper, G.; Jenthra, S.; Bolm, C.; Leitner, W. Factors governing the catalytic insertion of CO2 into arenes–A DFT case study for Pd and Pt phosphane sulfonamido complexes. Chemistry–A European Journal 2022, 28 (23), e202104375.
(4) Voit, G.; Jenthra, S.; Hölscher, M.; Weyhermüller, T.; Leitner, W. Reversible insertion of carbon dioxide at phosphine sulfonamido PdII–aryl complexes. Organometallics 2020, 39 (24), 4465-4473.
(5) Kemper, G.; Hölscher, M.; Leitner, W. Pd (II)-catalyzed carboxylation of aromatic C─ H bonds with CO2. Science Advances 2023, 9 (5), eadf2966.
(6) Kuß, D. A.; Hölscher, M.; Leitner, W. Combined Computational and Experimental Investigation on the Mechanism of CO2 Hydrogenation to Methanol with Mn-PNP-Pincer Catalysts. ACS Catalysis 2022, 12, 15310-15322.
(7) Kuß, D. A.; Hölscher, M.; Leitner, W. Hydrogenation of CO2 to Methanol with Mn‐PNP‐Pincer Complexes in the Presence of Lewis Acids: the Formate Resting State Unleashed. ChemCatChem 2021, 13 (14), 3319-3323.
(8) Singh, A.; Kemper, G.; Weyhermueller, T.; Kaeffer, N.; Leitner, W. Activated Mn‐MACHO Complexes Form Stable CO2 Adducts. Chemistry–A European Journal 2023, e202303438.

