Dr. Olaf Rüdiger - Spectroelectrochemistry
- Dr. Olaf Rüdiger
- Group leader
- Inorganic Spectroscopy
- +49 (0)208 306 - 3880
- olaf.ruediger(at)cec.mpg.de
- Room: 511
Vita
| B.Sc. | Universidad de Valencia, Spain (2003) | |
| M.Sc. | Universidad Autónoma de Madrid, Spain (2006) | |
| Ph.D. | Universidad Autónoma de Madrid and Instituto de Catálisis y Petroleoquímica (CSIC), Spain (2009) | |
| Research visits | University of Oxford, UK (Prof. Fraser Armstrong) (2005) and Texas A&M University, USA (Prof. Marcetta Darensbourg) | |
| Postdoc | MPI for Bioinorganic Chemistry; today: MPI CEC (2009-2013) | |
| Group leader | MPI CEC (since 2013) |
Publications
Full publications list | ORCID | ResearcherID | Google Scholar Profile
Selected MPI CEC publications
- Erbe, A., Tesch, M. F., Rüdiger, O., Kaiser, B., DeBeer, S., Rabe, M. (2023). Operando studies of Mn oxide based electrocatalysts for the oxygen evolution reaction. Physical Chemistry Chemical Physics, (40), 26933-27894. doi:10.1039/d3cp02384b.
- Alkan, B., Braun, M., Landrot, G., Rüdiger, O., Andronescu, C., DeBeer, S., Schulz, C., Wiggers, H. (2022). Spray-flame-synthesized Sr- and Fe-substituted LaCoO3 perovskite nanoparticles with enhanced OER activities. Journal of Materials Science, (57), 18923-18936. doi:10.1007/s10853-022-07738-z.
- Yu, M., Weidenthaler, C., Wang, Y., Budiyanto, E., Sahin, E. O., Chen, M., DeBeer, S., Rüdiger, O., Tueysuez, H. (2022). Surface Boron Modulation on Cobalt Oxide Nanocrystals for Electrochemical Oxygen Evolution Reaction. Angewandte Chemie, International Edition in English, (61): e202211543, pp. 1-12. doi:10.1002/anie.202211543.
- Xiang, W., Yang, N., Li, X., Linnemann, J., Hagemann, U., Ruediger, O.,Heidelmann,M.; Falk, T., Aramani, M., DeBeer, S., Muhler, M.,Tschulik, K., Li, T.. (2022). 3D atomic-scale imaging of mixed Co-Fe spinel oxide nanoparticles during oxygen evolution reaction. Nature Communications, (xx), 1-14. doi:10.1038/s41467-021-277.
- Czastka, K., Alsheikh Oughli, A., Rüdiger, O. ,DeBeer, S. (2022). Enzymatic X-ray Absorption Spectroelectrochemistry. Faraday Discussions, (234), 214-231. doi:10.1039/D1FD00079A.
- Levin, N., Casadevall, C., Cutsail III, G. E., Lloret-Fillol, J., DeBeer, S., Rüdiger, O. (2022). XAS and EPR in Situ Observation of Ru(V) Oxo Intermediate in a Ru Water Oxidation Complex**. CHEMELECTROCHEM, (9): e202101271, pp. 1-4. doi:10.1002/celc.202101271.
- Czastka, K., Alsheikh Oughli, A., Rüdiger, O., DeBeer, S. (2021). Enzymatic X-ray Absorption Spectroelectrochemistry. Faraday Discussions, (xx), 1-14. doi:10.1039/x0xx00000x.
- Martini, M. A., Rüdiger, O., Breuer, N., Nöring, B., DeBeer, S., Rodriguez-Macia, P., Birrell, J. (2021). The Nonphysiological Reductant Sodium Dithionite and [FeFe] Hydrogenase: Influence on the Enzyme Mechanism. Journal of the American Chemical Society, (xx), xx-xx. doi:10.1021/jacs.1c07322.
- Gil-Sepulcre, M., Lindner, J. O., Schindler, D., Velasco, L., Moonshiram, D., Rüdiger, O., De Beer, S., Stepanenko, V., Solano, E., Würthner, F., Llobet, A. (2021). Surface-Promoted Evolution of Ru-bda Coordination Oligomers Boosts the Efficiency of Water Oxidation Molecular Anodes. Journal of the American Chemical Society, 143(30), 11651-11661. doi:10.1021/jacs.1c04738.
- Budiyanto, E., Zerebecki, S., Weidenthaler, C., Kox, T., Kenmoe, S., Spohr, E., DeBeer, S., Rüdiger, O., Reichenberger, S., Barcikowski, S., Tuysuz, H., (2021). Impact of Single-Pulse, Low-Intensity Laser Post-Processing on Structure and Activity of Mesostructured Cobalt Oxide for the Oxygen Evolution Reaction. ACS applied materials & interfaces (13), 51962-51973 doi:10.1021/acsami.1c08034.
- Hardt, S., Stapf, S., Filmon, D. T., Birrell, J. A., Rüdiger, O., Fourmond, V., Léger, C.; Plumeré, N. (2021) Reversible H-2 oxidation and evolution by hydrogenase embedded in a redox polymer film. Nature Catalysis, 4(3), 251-258. doi:10.1038/s41929-021-00586-1.
- Oughli, A.A., Hardt, S., Rüdiger, O., Birrell, J.A., Plumeré, N. (2020). Reactivation of sulfide-protected [FeFe] hydrogenase in a redox-active hydrogel Chemical Communications 56(69), 9958-9961. https://doi.org/10.1039/D0CC03155K
- Budiyanto, E., Yu, M., Chen, M., DeBeer, S., Rüdiger, O., Tüysüz, H. (2020). Tailoring Morphology and Electronic Structure of Cobalt Iron Oxide Nanowires for Electrochemical Oxygen Evolution Reaction ACS Applied Energy Materials 3(9), 8583-8594. https://doi.org/10.1021/acsaem.0c01201
- Levin, N., Peredkov, S., Weyhermüller, T., Rüdiger, O., Pereira, N.B., Grötzsch, D., Kalinko, A., DeBeer, S. (2020). Ruthenium 4d-to-2p X-ray Emission Spectroscopy: A Simultaneous Probe of the Metal and the Bound Ligands Inorganic Chemistry 59(12), 8272-8283. https://doi.org/10.1021/acs.inorgchem.0c00663
- Chongdar, N., Pawlak, K., Rüdiger, O., Reijerse, E.J., Rodríguez-Maciá, P., Lubitz, W., Birrell, J.A., Ogata, H. (2020). Spectroscopic and biochemical insight into an electron-bifurcating [FeFe] hydrogenase Journal of Biological Inorganic Chemistry 25(1), 135-148. https://doi.org/10.1007/s00775-019-01747-1
- Kutin, Y., Cox, N., Lubitz, W., Schnegg, A., Rüdiger, O. (2019). In Situ EPR Characterization of a Cobalt Oxide Water Oxidation Catalyst at Neutral pH Catalysts 9(11), 926. https://doi.org/10.3390/catal9110926
- Al Samarai, M., Hahn, A.W., Askari, A.B., Cui, Y.-T., Yamazoe, K., Miyawaki, J., Harada, Y., Rüdiger, O., DeBeer, S. (2019). Elucidation of Structure-Activity Correlations in a Nickel-Manganese Oxide OER Catalyst by Operando Ni L-edge XAS and 2p3d RIXS ACS Applied Materials and Interfaces 11(42), 38595-38605. https://doi.org/10.1021/acsami.9b06752
- Rodríguez-Maciá, P., Kertess, L., Burnik, J., Birrell, J.A., Hofmann, E., Lubitz, W., Happe, T., Rüdiger, O. (2019). His-ligation to the [4Fe-4S] sub-cluster tunes the catalytic of [FeFe] hydrogenase Journal of the American Chemical Society 141, 472 481. https://doi.org/10.1021/jacs.8b11149
- Shankar, S., Peters, M., Steinborn, K., Krahwinkel, B., Sönnichsen, F.D., Grote, D., Sander, W., Lohmiller, T., Rüdiger, O., Herges, R. (2018). Light-controlled switching of the spin state of iron(III) Nature Communications 9, 4750. https://doi.org/10.1038/s41467-018-07023-1
- Rodriguez-Maciá, P., Reijerse, E.J., van Gastel, M., DeBeer, S., Lubitz, W., Rüdiger, O., Birrell, J.A. (2018). Sulfide Protects [FeFe] Hydrogenases from O2 Journal of the American Chemical Society 140(30), 9346-9350. https://doi.org/10.1021/jacs.8b04339
- Oughli, A.A., Vélez, M., Birrell, J., Schuhmann, W., Lubitz, W., Plumeré, N., Rüdiger, O. (2018). Viologen-modified Electrodes for Protection of Hydrogenases from High Potential Inactivation while Performing H2 Oxidation at Low Overpotential Dalton Transactions 47, 10685 10691. https://doi.org/10.1039/C8DT00955D
- Oughli, A.A., Ruff, A., Boralugodage, N.P., Rodríguez-Maciá, P., Plumere, N., Lubitz, W., Shaw, W.J., Schuhmann, W., Rüdiger, O. (2018). Dual properties of a hydrogen oxidation Ni-catalyst entrapped within a polymer promote self-defense against oxygen Nature Communications 9, 864. https://doi.org/10.1038/s41467-018-03011-7
- Kertess, L., Adamska-Venkatesh, A., Rodríguez-Maciá, P., Rüdiger, O., Lubitz, W., Happe, T. (2017). Influence of the [4Fe-4S] cluster coordinating cysteines on active site maturation and catalytic properties of C. reinhardtii [FeFe]-hydrogenase Chemical Science 8(12), 8127-8137. https://doi.org/10.1039/c7sc03444j
- Sommer, C., Adamska-Venkatesh, A., Pawlak, K., Birrell, J.A., Rüdiger, O., Reijerse, E.J., Lubitz, W. (2017). Proton Coupled Electronic Rearrangement within the H-Cluster as an Essential Step in the Catalytic Cycle of [FeFe] Hydrogenases Journal of the American Chemical Society 139(4), 1440 -1443. https://doi.org/10.1021/jacs.6b12636
- Birrell, J.A., Rüdiger, O., Reijerse, E.J., Lubitz, W. (2017). Semisynthetic Hydrogenases Propel Biological Energy Research into a New Era Joule 1(1), 61-76. https://doi.org/10.1016/j.joule.2017.07.009
- Rodríguez-Maciá, P., Reijerse, E., Lubitz, W., Birrell, J.A., Rüdiger, O. (2017). Spectroscopic Evidence of Reversible Disassembly of the [FeFe] Hydrogenase Active Site Journal of Physical Chemistry Letters 8(16), 3834-3839. https://doi.org/10.1021/acs.jpclett.7b01608
- Engelbrecht V., Rodríguez-Maciá P., Esselborn J., Sawyer A., Hemschemeier A., Rüdiger O., Lubitz W., Winkler M., Happe T. (2017). The structurally unique photosynthetic Chlorella variabilis NC64A hydrogenase does not interact with plant-type ferredoxins Biochimica et Biophysica Acta (BBA) – Bioenergetics 1858(9), 771-778. https://doi.org/10.1016/j.bbabio.2017.06.004
- Kertess L., Wittkamp F., Sommer C., Esselborn J., Rüdiger O., Reijerse E.J., Hofmann E., Lubitz W., Winkler M., Happe T., Apfel U.-P. (2017). Chalcogenide substitution in the [2Fe] cluster of [FeFe]-hydrogenases conserves high enzymatic activity Dalton Transactions 46, 16947-16958. https://doi.org/10.1039/c7dt03785f
- Rodríguez-Maciá, P., Pawlak, K., Rüdiger, O., Reijerse, E.J., Lubitz, W., Birrell, J.A. (2017). Intercluster Redox Coupling Influences Protonation at the H-cluster in [FeFe] Hydrogenases Journal of the American Chemical Society 139(42), 15122-15134. https://doi.org/10.1021/jacs.7b08193
- Lampret, O., Adamska-Venkatesh, A., Konegger, H., Wittkamp, F., Apfel, U.-P., Reijerse, E.J., Lubitz, W., Rüdiger, O., Happe, T., Winkler, M. (2017). Interplay between CN– Ligands and the Secondary Coordination Sphere of the H-Cluster in [FeFe]-Hydrogenases Journal of the American Chemical Society 139(50), 18222-18230. https://doi.org/10.1021/jacs.7b08735
- Rodríguez-Maciá, P., Birrell, J.A., Lubitz, W., Rüdiger, O. (2017). Electrochemical Investigations on the Inactivation of the [FeFe] Hydrogenase from Desulfovibrio desulfuricans by O2 or Light under Hydrogen-Producing Conditions ChemPlusChem 82(4), 540-545. https://doi.org/10.1002/cplu.201600508
- Rodríguez-Maciá, P., Priyadarshani, N., Dutta, A., Weidenthaler, C., Lubitz, W., Shaw, W.J., Rüdiger, O. (2016). Covalent Attachment of the Water-insoluble Ni(PCy2NPhe2) Electrocatalyst to Electrodes Showing Reversible Catalysis in Aqueous Solution Electroanalysis 28(10), 2452-2458. https://doi.org/10.1002/elan.201600306
- Birrell, J.A., Wrede, K., Pawlak, K., Rodríguez-Maciá, P., Rüdiger, O., Reijerse, E.J., Lubitz, W. (2016). Artificial Maturation of the Highly Active Heterodimeric [FeFe] Hydrogenase from Desulfovibrio desulfuricans ATCC 7757 Israel Journal of Chemistry 56(9-10), 852-863. https://doi.org/10.1002/ijch.201600035
Spectroelectrochemistry
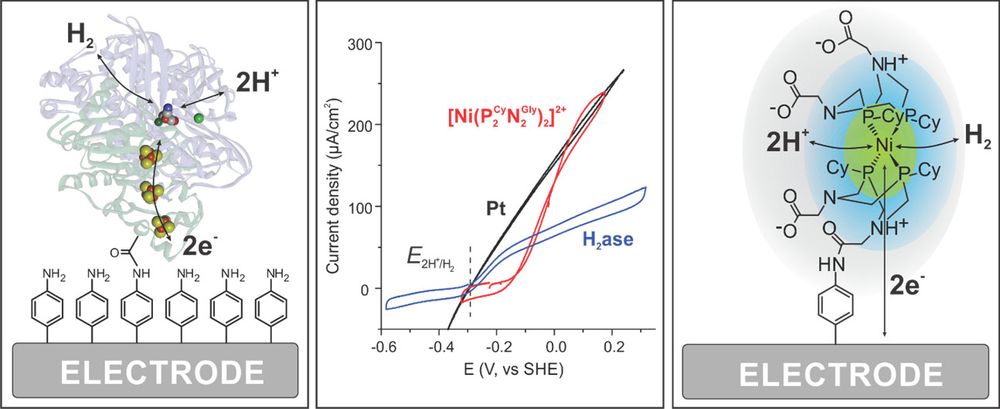
Proteins and catalysts on electrodes
Our group’s research is focused on the electrochemical study of hydrogen cycling catalysts, more specifically hydrogenases and bio-inspired molecular catalysts. Hydrogenases are the most efficient noble-metal-free catalysts for H2 production or oxidation. These enzymes use earth abundant metals in the active site (as Ni or Fe) and work at almost no overpotential under mild conditions.1 Chemists have been studying these enzymes to understand their unique properties and learn how to design bio-inspired catalysts avoiding the use of noble metals. The goal is to have efficient catalysts for implementation in energy conversion devices that could be employed to efficiently store energy from discontinuous renewable sources in chemical bonds (e.g. molecular H2) as a fuel and recover that energy when required.
Protein film electrochemistry (PFE) has been proven as a highly useful technique to study immobilized hydrogenases to gain fundamental information about the enzyme kinetics and their sensitivity towards inhibitors such as O2 and CO.2-3 Much less is known about homogeneous bio-inspired catalysts. In recent years, there has been significant improvement in the development of bio-inspired synthetic catalysts based on earth abundant metals like Fe, Ni or Co. These catalysts have so far been studied using methods very different from the ones used with enzymes. We have shown that we can immobilize some of these catalysts on electrodes and characterize them under conditions mimicking those of a device e.g. a fuel cell or a H2 producing electrode. We can now also compare the activity of the bio-inspired catalysts for H2 oxidation with the enzyme (Figure 1).4-5
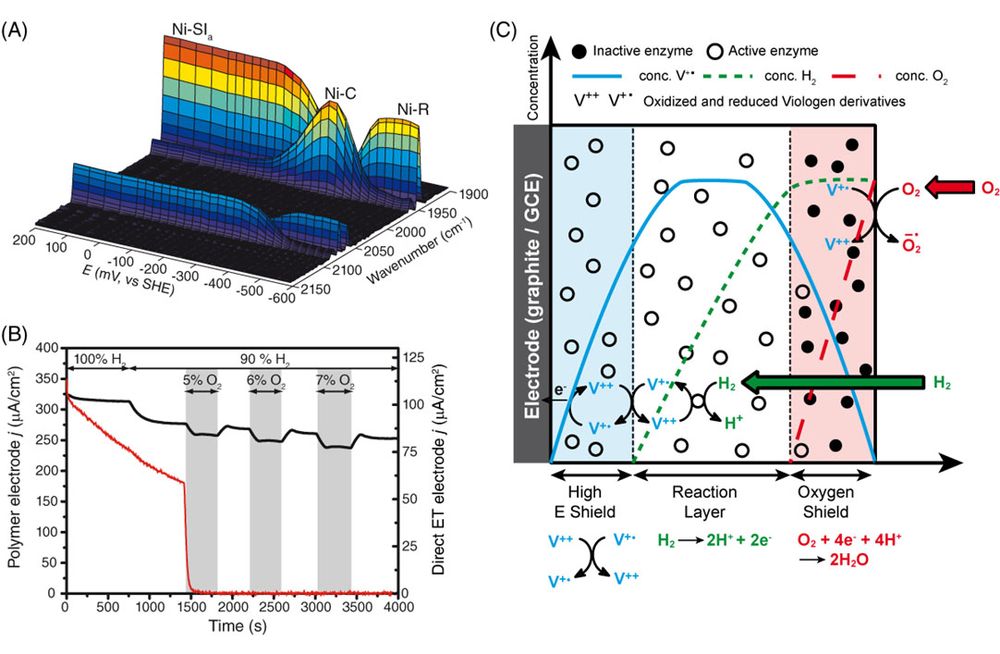
Hydrogenases in redox hydrogels
When designing an immobilization strategy for a catalyst on an electrode, it is possible to improve the electron transfer between catalyst and electrode and the stability of the catalytic currents. But for delicate catalysts as hydrogenases, sensible to oxidative inactivation, directly interfacing the catalyst with the electrode leads to a rapid loss of electrocatalytic current when the catalyst is exposed to a harsh environment, as is found in an operating fuel cell. In the last years, together with our colleagues at the Ruhr University in Bochum, Wolfgang Schuhmann and Nicolas Plumeré, we have specifically designed redox polymers to protect sensitive hydrogenases from oxidative inactivation and demonstrated the protection mechanism (Figure 2).6-8
In situ spectroelectrochemistry
Immobilizationof catalysts on electrode surfaces allows precise redox control of theimmobilized molecules and measurements of catalytic currents. On the otherhand, electrochemistry alone does not provide information about the electronicstructure of the immobilized catalysts. Therefore, we combine electrochemistrywith spectroscopy to obtain information from immobilized catalysts underturnover conditions. Combination of IR spectroscopy with PFE can be achievedusing surface-enhanced infrared absorption spectroscopy (SEIRA).9
In collaborationwith the Savitsky and Cox groups, we are currently developing insitu spectroelectrochemical cells for EPR, pulse and continuous wave, X and Q-band to detect paramagneticspecies participating in catalysis from electrode immobilized catalysts.
rrent when the catalyst is exposedto a harsh environment, as is found in an operating fuel cell. In the lastyears, together with our colleagues at the Ruhr University in Bochum, WolfgangSchuhmann and Nicolas Plumeré, we havespecifically designed redox polymersto protect sensitive hydrogenases from oxidative inactivation and demonstratedthe protection mechanism (Figure 2).6-8
References
- Lubitz, W.; Ogata, H.; Ruediger, O.; Reijerse, E., Hydrogenases. Chem. Rev. 2014, 114 (8), 4081-4148.
- Rodríguez-Maciá, P.; Birrell, J. A.; Lubitz, W.; Rüdiger, O., Electrochemical Investigations on the Inactivation of the [FeFe] Hydrogenase from Desulfovibrio desulfuricans by O2 or Light under Hydrogen-Producing Conditions. ChemPlusChem 2016, DOI: 10.1002/cplu.201600508
- Adamska, A.; Silakov, A.; Lambertz, C.; Rüdiger, O.; Happe, T.; Reijerse, E.; Lubitz, W., Identification and Characterization of the 'Super-Reduced' State of the H-Cluster in [FeFe] Hydrogenase: A New Building Block for the Catalytic Cycle? Angew. Chem. Int. Ed. 2012, 51 (46), 11458-11462.
- Rodriguez-Macia, P.; Priyadarshani, N.; Dutta, A.; Weidenthaler, C.; Lubitz, W.; Shaw, W. J.; Rudiger, O., Covalent Attachment of the Water-insoluble Ni((P2N2Phe)-N-Cy)(2) Electrocatalyst to Electrodes Showing Reversible Catalysis in Aqueous Solution. Electroanalysis 2016, 28 (10), 2452-2458.
- Rodriguez-Macia, P.; Dutta, A.; Lubitz, W.; Shaw, W. J.; Rudiger, O., Direct comparison of the performance of a bio-inspired synthetic nickel catalyst and a [NiFe]-hydrogenase, both covalently attached to electrodes. Angew. Chem. Int. Ed. Engl. 2015, 54 (42), 12303-7.
- Fourmond, V.; Stapf, S.; Li, H.; Buesen, D.; Birrell, J.; Rüdiger, O.; Lubitz, W.; Schuhmann, W.; Plumeré, N.; Léger, C., Mechanism of Protection of Catalysts Supported in Redox Hydrogel Films. J. Am. Chem. Soc. 2015.
- Oughli, A. A.; Conzuelo, F.; Winkler, M.; Happe, T.; Lubitz, W.; Schuhmann, W.; Rudiger, O.; Plumere, N., A Redox Hydrogel Protects the O-2-Sensitive FeFe -Hydrogenase from Chlamydomonas reinhardtii from Oxidative Damage. Angew. Chem. Int. Ed. 2015, 54 (42), 12329-12333.
- Plumeré, N.; Rüdiger, O.; Oughli, A. A.; Williams, R.; Vivekananthan, J.; Pöller, S.; Schuhmann, W.; Lubitz, W., A redox hydrogel protects hydrogenase from high-potential deactivation and oxygen damage. Nat Chem 2014, 6 (9), 822-827.
- Gutiérrez-Sanz, O.; Marques, M.; Pereira, I. A. C.; de Lacey, A. L.; Lubitz, W.; Rüdiger, O., Orientation and Function of a Membrane Bound Enzyme Monitored by Electrochemical Surface Enhanced Infrared Absorption Spectroscopy. J. Phys. Chem. Lett. 2013, 4, 2794-2798.

