Storing energy in chemicals requires the conversion of inert small molecules, such as CO(2) or H+. The activation and catalytic transformation of these molecules often rely on highly reduced states at metal complexes. These reduced complexes are classically accessed through multiple reduction steps from higher oxidation states, a strategy that hence pends on a rationalized understanding of the reductive behavior.
A collaborative study led by Dr. Nicolas Kaeffer and Prof. Walter Leitner between the groups at MPI-CEC and RWTH Aachen now dissects these reduction processes in the case of rhodium complexes built on a popular class of chelating diphosphine ligands.
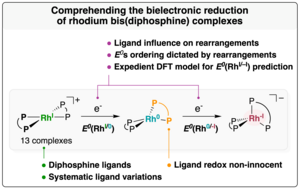
The study investigates across a series of parameterized rhodium bis(diphosphine) complexes the two-electron reduction to the low-valent Rh(–I) state, through combined experimental and computational approaches. The doctoral researcher Anne-Christine Kick and the team could evidence how the gradual rearrangement of the coordination sphere from the +I to the –I oxidation state affects the reduction behavior, which is manifested by either a stepwise, monoelectronic event or a bielectronic transition. To further address the impact of the diphosphine structure on the reduction, the researchers developed a methodology to estimate the reduction potential of the investigated complexes from a parameter expediently computed on related in silico models.
Overall, the work contributes to understanding the factors underpinning the behaviour in bielectronic reductions and thusly to gain control over electrochemical properties by ligand design. These insights are of interest not only for chemistry related to low-valent rhodium but more generally for the development of novel molecular catalysts in redox applications.
Original publication: Anne-Christine Kick, Thomas Weyhermüller, Markus Hölscher, Nicolas Kaeffer, and Walter Leitner (2024) Understanding Ligand Effects on Bielectronic Transitions: Chemo- and Electroreduction of Rhodium Bis(Diphosphine) Complexes to Low Oxidation States. Angewandte Chemie International Edition, Accepted Article DOI: 10.1002/anie.202408356