Dr. Christina Römelt - Chemical Synthesis
- Dr. Christina Römelt
- Group leader
- Chemical Synthesis
- Inorganic Spectroscopy
- +49 (0)208 306 - 3694
- christina.roemelt(at)cec.mpg.de
- Room: 512
Vita
| Studies (Chemistry) | University of Bonn (2003-2008) |
| Diploma (organic Chemistry) | University of Bonn (2008) |
| Dr. rer. nat. (organic Chemistry) | University of Bonn (2008-2010), University of Mainz (2010-2013) |
| Research project | Novartis, Basel (2013) |
| Scientific staff | MPI CEC (2014-2017) |
| Group leader | Chemical Synthesis, MPI CEC (since 2017) |
Publications
Full publications list | ORCID
Selected MPI CEC publications
- Tarrago, M., Römelt, C., Nehrkorn, J., Schnegg, A., Neese, F., Bill, E., Ye, S. (2021). Experimental and Theoretical Evidence for an Unusual Almost Triply Degenerate Electronic Ground State of Ferrous Tetraphenylporphyrin. Inorganic chemistry. doi:10.1021/acs.inorgchem.1c00031.
- Berkefeld, A., Roemelt, M., Römelt, C., Schubert, H., Jeschke, G. (2020). Modulating Effect of Ligand Charge on the Electronic Properties of 2Ni–2S Structures and Implications for Biological 2M–2S Sites Inorganic Chemistry 59(23),17234–17243. https://doi.org/10.1021/acs.inorgchem.0c02467
- Römelt, C., Weyhermüller, T., Wieghardt, K. (2019). Structural characteristics of redox-active pyridine-1,6-diimine complexes: Electronic structures and ligand oxidation levels Coordination Chemistry Reviews 380, 287-317. https://doi.org/10.1016/j.ccr.2018.09.018
- Wang, M., Römelt, C., Weyhermüller, T., Wieghardt, K. (2019). Coordination Modes, Oxidation, and Protonation Levels of 2,6-Pyridinediimine and 2,2′:6′,2′́-Terpyridine Ligands in New Complexes of Cobalt, Zirconium, and Ruthenium. An Experimental and Density Functional Theory Computational Study Inorganic Chemistry 58(1), 121 132. https://doi.org/10.1021/acs.inorgchem.8b01949
- Römelt, C., Ye, S., Bill, E., Weyhermuller, T., van Gastel, M., Neese, F. (2018). Electronic Structure and Spin Multiplicity of Iron Tetraphenylporphyrins in Their Reduced States as Determined by a Combination of Resonance Raman Spectroscopy and Quantum Chemistry Inorganic Chemistry 57(4), 2141-2148. https://doi.org/10.1021/acs.inorgchem.7b03018
- Römelt, C., Song, J.S., Tarrago, M., Rees, J.A., van Gastel, M., Weyhermüller, T., DeBeer, S., Bill, E., Neese, F., Ye, S. (2017). Electronic Structure of a Formal Iron(0), Porphyrin Complex Relevant to CO2 Reduction Inorganic Chemistry 56(8), 4745-4750. https://doi.org/10.1021/acs.inorgchem.7b00401
Chemical Synthesis
Due to the vast employment of carbon-based fuels in combustion processes, the amount of CO2 in the atmosphere has increased by almost 40% over the last hundred years. The research of my group is focused on the reconversion of CO2 into building blocks which can be employed for further use (formaldehyde, formic acid, oxalic acid, methanol, CO). Due to its thermodynamic stability, the electrochemical reduction of CO2 to the CO2●– radical (E0´= –1.90 V) has to be facilitated by using suitable catalysts. Iron tetraphenylporphyrins (FeTPP´s) in the presence of external or internal proton sources are reported to exhibit one of the highest catalytic activities and turnover frequencies observed so far.1-5
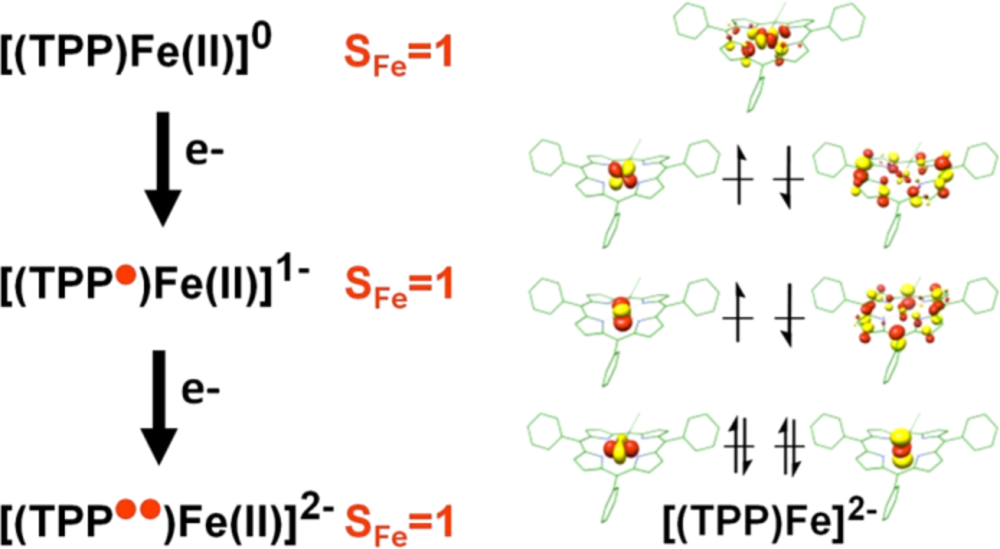
Since the nature of the electronic ground state of the catalytically active species [FeTPP]2– has been controversially discussed for decades, we focused on the elucidation the electronic structure of these compounds by employing a combination of spectroscopy and theory, with both Mössbauer and XAS spectroscopic results supporting ligand reduction (Fig. 1).6
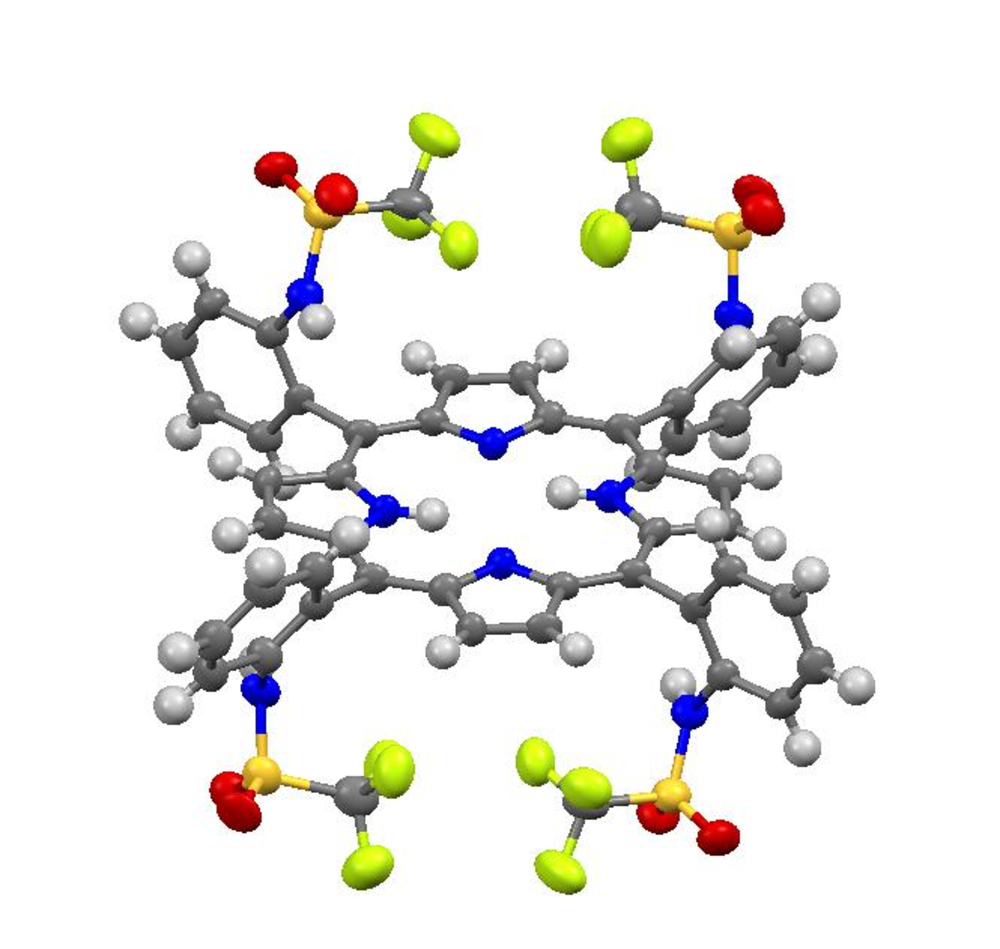
Furthermore, the synthesis of novel catalysts and investigation of their efficiency in the electrochemical CO2 reduction are topic of our current research. Our approach is the design of new TPP systems providing optimized substituents to a) stabilize the CO2-Fe moiety by strong hydrogen bonds and b) to donate protons more easily by using substituents with a low pKa value. Fe-CO2 adduct stabilization might lead to an even greater enhancement of the catalytic activity and/or the opportunity to stabilize this species for further characterization which hopefully gives insight into this very crucial step in catalysis. In a first attempt, we have synthesized a new TPP ligand system carrying four sulfonamide groups (Fig. 2). Iron insertion will then render a promising candidate for catalysis.
References
1. I. Bhugun, D. Lexa and J. M. Saveant, J. Am. Chem. Soc. 1996, 118, 1769 - 1776.
2. M. Hammouche, D. Lexa, J. M. Saveant and M. Momenteau, J. Electroanal. Chem. Interfacial Electrochem. 1988, 249, 347-351.
3. C. Costentin, S. Drouet, G. Passard, M. Robert and J. M. Saveant, J. Am. Chem. Soc. 2013, 135, 9023-9031.
4. C. Costentin, G. Passard, M. Robert and J. M. Saveant, P. Natl. Acad. Sci 2014, 111, 14990 - 14994.
5. C. Costentin, G. Passard, M. Robert and J. M. Saveant, J. Am.Chem. Soc. 2014, 136, 11821-11829.
6. C. Roemelt, J. Song, M. Tarrago, J. A. Rees, M. van Gastel, T. Weyhermueller, S. DeBeer, E. Bill, F. Neese and S. Ye, Inorg. Chem. 2017, 56, 4745-4750.