Dr. Thomas Weyhermüller - Chemical Synthesis, X-ray structure analysis
- Dr. Thomas Weyhermüller
- Group leader
- Chemical Synthesis, X-ray structure analysis
- Inorganic Spectroscopy
- +49 (0)208 306 - 3652
- thomas.weyhermueller(at)cec.mpg.de
- Room: 514
Vita
Publications
Full publications list | ORCID | ResearcherID
Selected MPI CEC publications
- Durin, G., Lee, M., Pogany, M. A., Weyhermüller, T., Kaeffer, N., Leitner, W. (2023). Hydride-Free Hydrogenation: Unraveling the Mechanism of Electrocatalytic Alkyne Semihydrogenation by Nickel-Bipyridine Complexes. JOURNAL OF THE AMERICAN CHEMICAL SOCIETy, 145(31), 17103-17111. doi:10.1021/jacs.3c03340.
- Chang, W.-C., Randel, H., Weyhermüller, T., Auer, A. A. A., Fares, C., Werlé, C. (2023). A Cooperative Rhodium/Secondary Phosphine Oxide [Rh/P(O)nBu(2)] Template for Catalytic Hydrodefluorination of Perfluoroarenes. Angewandte Chemie, International Edition in English, (62): e202219127, pp. 1-10. doi:10.1002/anie.202219127.
- Yogendra, S., Wilson, D. W. N., Hahn, A. W., Weyhermüller, T., Van Stappen, C., Holland, P., DeBeer, S. (2023). Sulfur-Ligated [2Fe-2C] Clusters as Synthetic Model Systems for Nitrogenase. INORGANIC CHEMISTRY, 62(6), 2663-2671. doi:10.1021/acs.inorgchem.2c03693.
- Antico, E., Leutzsch, M., Wessel, N., Weyhermüller, T., Werlé, C., Leitner, W. (2022). Selective oxidation of silanes into silanols with water using [MnBr(CO)(5)] as a precatalyst. Chemical Science, 14(1), 54-60. doi:10.1039/d2sc05959b.
- Harihar, S., Mone, N., Satpute, S. K., Chadar, D., Chakravarty, D., Weyhermüller, T., Butcher, R. J., Salunke-Gawali, S. (2022). Metal complexes of a pro-vitamin K3 analog phthiocol (2-hydroxy-3-methylnaphthalene-1,4-dione): synthesis, characterization, and anticancer activity. Dalton Transactions, (51), 17338-17353. doi:10.1039/d2dt02748h.
- Chugh, V., Chatterjee, B., Chang, W.-C., Cramer, H. H., Hindemith, C., Randel, H., Weyhermüller, T., Fares, C., Werle, C. (2022). An Adaptive Rhodium Catalyst to Control the Hydrogenation Network of Nitroarenes. Angewandte Chemie, International Edition in English, (61): e202205515, pp. 1-10. doi:10.1002/anie.202205515.
- Henthorn, J. T., Cutsail III, G. E., Weyhermüller, T., DeBeer, S. (2022). Stabilization of intermediate spin states in mixed-valent diiron dichalcogenide complexes. Nature Chemistry, (14), 328-333. doi:10.1038/s41557-021-00853-5.
- Shit, M., Maity, S., Bera, S., Weyhermüller, T., Ghosh, P. (2022). Coordination of o-benzosemiquinonate, o-iminobenzosemiquinonate, 4, 4 '-di-tert-butyl-2,2 '-bipyridine and 1,10-phenanthroline anion radicals to oxidovanadium(IV) (vol 40, pg 10305, 2016). New Journal of Chemistry, 46(7), 3521-3521. doi:10.1039/d2nj90016e.
- Chang, W.-C., Deufel, F., Weyhermüller, T., Fares, C., Werlé, C. (2021). Rhodium(I) complexes derived from tris(isopropyl)-azaphosphatrane-controlling the metal-ligand interplay. RSC Advances, 11(59), 37383-37391. doi:10.1039/d1ra07126b.
- Kinzel, N. W., Demirbas, D., Bill, E., Weyhermüller, T., Werlé, C., Kaeffer, N., Leitner, W. (2021). Systematic Variation of 3d Metal Centers in a Redox-Innocent Ligand Environment: Structures, Electrochemical Properties, and Carbon Dioxide Activation. Inorganic Chemistry, (xx), xx-xx. doi:10.1021/acs.inorgchem.1c02909.
- Perdomenico, J., Levin, N., Fierro, A. C., Chernek, O. A. C., Weyhermüller, T., Slep, L. D. (2021). A New Member of the Growing Family of Interconvertible {RuNO}(6,7,8) Species. Redox and Acid-Base Characterization of [Ru((CH(2)py)(2)Me[9]aneN(3))(NO)](n+). European Journal of Inorganic Chemistry, 4842-4855. doi:10.1002/ejic.202100753.
- Chatterjee, B., Jena, S., Chugh, V., Weyhermüller, T., Werle, C. (2021). A Molecular Iron-Based System for Divergent Bond Activation: Controlling the Reactivity of Aldehydes. ACS CATALYSIS, 11(12), 7176-7185. doi:10.1021/acscatal.1c00733.
- Shit, M., Maity, S., Bera, S., Kumar Mudi, P., Biswas, B., Weyhermüller, T., Ghosh, P. (2021). Nickel (II) di-aqua complex containing water cluster: Synthesis, X-ray structure and catecholase activity New Journal of Chemistry, 45, 2221--2227 https://doi.org/10.1039/D0NJ05238H
- Voit, G., Jenthra, S., Hölscher, M., Weyhermüller, T., Leitner, W. (2020). Reversible Insertion of Carbon Dioxide at Phosphine Sulfonamido PdII–Aryl Complexes Organometallics 39(24), 4465-4473. https://doi.org/10.1021/acs.organomet.0c00560
- Levin, N., Peredkov, S., Weyhermüller, T., Rüdiger, O., Pereira, N.B., Grötzsch, D., Kalinko, A., DeBeer, S. (2020). Ruthenium 4d-to-2p X-ray Emission Spectroscopy: A Simultaneous Probe of the Metal and the Bound Ligands Inorganic Chemistry 59(12), 8272-8283. https://doi.org/10.1021/acs.inorgchem.0c00663
- Cramer, H.H., Chatterjee, B., Weyhermüller, T., Werlé, C., Leitner, W. (2020). Controlling the Product Platform of Carbon Dioxide Reduction: Adaptive Catalytic Hydrosilylation of CO2 Using a Molecular Cobalt(II) Triazine Complex Angewandte Chemie International Edition 59(36), 15674-15681. https://doi.org/10.1002/anie.202004463
- Erken, C., Hindemith, C., Weyhermüller, T., Hölscher, M., Werlé, C., Leitner, W. (2020). Hydroamination of Aromatic Alkynes to Imines Catalyzed by Pd(II)–Anthraphos Complexes ACS Omega 5(15), 8912-8918. https://doi.org/10.1021/acsomega.0c00562
- Dutta, D., Kundu, S., Weyhermüller, T., Ghosh, P. (2020). Metal promoted conversion of aromatic amines to ortho-phenylenediimine derivatives by a radical coupling path Dalton Transactions 49(16), 5015-5019. https://doi.org/10.1039/D0DT00089B
- Dinda, S., Patra, S.C., Roy, S., Halder, S., Weyhermüller, T., Pramanik, K., Ganguly, S. (2020). Coligand driven diverse organometallation in benzothiazolyl-hydrazone derivatized pyrene: ortho vs peri C–H activation New Journal of Chemistry 44(4), 1407-1417. https://doi.org/10.1039/c9nj05088d
- Yogendra, S., Weyhermüller, T., Hahn, A.W., DeBeer, S. (2019). From Ylides to Doubly Yldiide-Bridged Iron(II) High Spin Dimers via Self-Protolysis Inorganic Chemistry 58(14), 9358-9367. https://doi.org/10.1021/acs.inorgchem.9b01086
- Kalläne, S.I., Hahn, A.W., Weyhermüller, T., Bill, E., Neese, F., DeBeer, S., van Gastel, M. (2019). Spectroscopic and Quantum Chemical Investigation of Benzene-1,2- dithiolate-Coordinated Diiron Complexes with Relevance to Dinitrogen Activation Inorganic Chemistry 58(8), 5111-5125. https://doi.org/10.1021/acs.inorgchem.9b00177
- Bhand, S., Landem D.N., Pereira, E., Gejji, S.P., Weyhermüller, T., Chakravarty, D., Puranik, V.G., Salunke-Gawali, S. (2019). Amphiphilic polypyridyl ruthenium complexes: Synthesis, Characterization and Aggregation studies Polyhedron 164, 96-107. https://doi.org/10.1016/j.poly.2019.02.035
- Römelt, C., Weyhermüller, T., Wieghardt, K. (2019). Structural characteristics of redox-active pyridine-1,6-diimine complexes: Electronic structures and ligand oxidation levels Coordination Chemistry Reviews 380, 287-317. https://doi.org/10.1016/j.ccr.2018.09.018
- Wang, M., Römelt, C., Weyhermüller, T., Wieghardt, K. (2019). Coordination Modes, Oxidation, and Protonation Levels of 2,6-Pyridinediimine and 2,2′:6′,2′́-Terpyridine Ligands in New Complexes of Cobalt, Zirconium, and Ruthenium. An Experimental and Density Functional Theory Computational Study Inorganic Chemistry 58(1), 121-132. https://doi.org/10.1021/acs.inorgchem.8b01949
- Erken, C., Kaithal, A., Sen, S., Weyhermüller, T., Hölscher, M., Werlé, C., Leitner, W. (2018). Manganese-catalyzed hydroboration of carbon dioxide and other challenging carbonyl groups Nature Communications 9, 4521. https://doi.org/10.1038/s41467-018-06831-9
- Kundu, S., Dutta, D., Maity, S., Weyhermüller, T., Ghosh, P. (2018). Proton-Coupled Oxidation of a Diarylamine: Amido and Aminyl Radical Complexes of Ruthenium(II) Inorganic Chemistry 57(19), 11948-11960. https://doi.org/10.1021/acs.inorgchem.8b01401
- Levin, N., Codesido N.O., Marcolongo, J.P., Alborés, Weyhermüller, T., Olabe, J.A., Slep, L.D. (2018). Remarkable Changes of the Acidity of Bound Nitroxyl (HNO) in the [Ru(Me3[9]aneN3)(L2)(NO)]n+ Family (n = 1-3). Systematic Structural and Chemical Exploration and Bioinorganic Chemistry Implications Inorganic Chemistry 57(19), 12270-12281. https://doi.org/10.1021/acs.inorgchem.8b01958
- Hahn, A.W., Van Kuiken, B.E., Chilkuri, V.G., Levin, N., Bill, E., Weyhermüller, T., Nicolaou, A., Miyawaki, J., Harada, Y., DeBeer, S. (2018). Probing the Valence Electronic Structure of Low-Spin Ferrous and Ferric Complexes Using 2p3d Resonant Inelastic X ray Scattering (RIXS) Inorganic Chemistry 57(37), 11918-11923. https://doi.org/10.1021/acs.inorgchem.8b01550
- Van Kuiken, B.E., Hahn, A.W., Nayyar, B., Schiewer, C.E., Lee, S.C., Meyer, F., Weyhermüller, T., Nicolaou, A., Cui, Y-T., Miyawaki, J., Hatada, Y., DeBeer, S. (2018). Electronic Spectra of Iron-Sulfur Complexes Measured by 2p3d RIXS Spectroscopy Inorganic Chemistry 57(12), 7355-7361. https://doi.org/10.1021/acs.inorgchem.8b01010
- Römelt, C., Ye, S., Bill, E., Weyhermüller, T., van Gastel, M., Neese, F. (2018). Electronic Structure and Spin Multiplicity of Iron Tetraphenylporphyrins in their Reduced States as Determined by a Combination of Resonance Raman Spectroscopy and Quantum Chemistry Inorganic Chemistry 57(4), 2141-2148. doi.org/10.1021/acs.inorgchem.7b03018
- Rees, J.A., Bjornsson, R., Kowalska, J.K., Lima, F.A., Schlesier, J., Sippel, D., Weyhermüller, T., Einsle, O., Kovacs, J.A., DeBeer, S. (2017). Comparative electronic structures of nitrogenase FeMoco and FeVco Dalton Transactions 46(8), 2445-2455. https://doi.org/10.1039/c7dt00128b
- Suturina, E.A., Nehrkorn, J., Zadrozny, J.M., Liu, J., Atanasov, M., Weyhermüller, T., Maganas, D., Hill, S., Schnegg, A., Bill, E., Long, J.R., Neese, F. (2017). Magneto-Structural Correlations in Pseudotetrahedral Forms of the [Co(SPh)4]2- Complex Probed by Magnetometry, MCD Spectroscopy, Advanced EPR Techniques, and ab Initio Electronic Structure Calculations Inorganic Chemistry 56(5), 3102-3118. https://doi.org/10.1021/acs.inorgchem.7b00097
- Chowdhury, A.R., Roy, B.G., Jana, S., Weyhermüller, T., Banerjee, P. (2017). A simple cleft shaped hydrazine-functionalized colorimetric new Schiff base chemoreceptor for selective detection of F- in organic solvent through PET signaling: Development of a chemoreceptor based sensor kit for detection of fluoride Sensors and Actuators B: Chemical 241, 706-715. https://doi.org/10.1016/j.snb.2016.10.095
- Römelt, C., Song, J.S., Tarrago, M., Rees, J.A., van Gastel, M., Weyhermüller, T., DeBeer, S., Bill, E., Neese, F., Ye, S. (2017). Electronic Structure of a Formal Iron(0),Porphyrin Complex Relevant to CO2 Reduction Inorganic Chemistry 56(8), 4745-4750. https://doi.org/10.1021/acs.inorgchem.7b00401
- Manikandamathavan V.M., Thangaraj M., Weyhermüller T., Parameswari R.P., Punitha V., Murthy N.N., Nair B.U. (2017). Novel mononuclear Cu(II) terpyridine complexes: Impact of fused ring thiophene and thiazole head groups towards DNA/BSA interaction, cleavage and antiproliferative activity on HepG2 and triple negative CAL-51 cell line European Journal of Medicinal Chemistry 135(28), 434-446. https://doi.org/10.1016/j.ejmech.2017.04.030
- Sabenya, G., Lázaro, L., Gambo, I., Martin-Diaconescu, V., Andris, E., Weyhermüller, T., Neese, F., Roithova, J., Bill, E., Lloret-Fillol, J., Costas, M. (2017). Generation, Spectroscopic, and Chemical Characterization of an Octahedral Iron(V)-Nitrido Species with a Neutral Ligand Platform Journal of the American Chemical Society 139(27), 9168-9177. https://doi.org/10.1021/jacs.7b00429
- Hahn, A.W., Van Kuiken, B.E., al Samarai, M., Atanasov, M., Weyhermüller, T., Cui, Y.T., Miyawaki, J., Harada, Y., Nicolaou, A., DeBeer, S. (2017). Measurement of the Ligand Field Spectra of Ferrous and Ferric Iron Chlorides Using 2p3d RIXS Inorganic Chemistry 56(14), 8203-8211. https://doi.org/10.1021/acs.inorgchem.7b00940
- Kowalska, J.K., Nayyar, B., Rees, J.A., Schiewer, C.E., Lee, S.C., Kovacs, J.A., Meyer, F., Weyhermüller, T., Otero, E., DeBeer, S. (2017). Iron L2,3-edge X-ray Absorption and X-ray Magnetic Circular Dichroism Studies of Molecular Iron Complexes with Relevance to the FeMoco and FeVco Active Sites of Nitrogenase Inorganic Chemistry 56(14), 8147-8158. https://doi.org/10.1021/acs.inorgchem.7b00852
- Khannam, M., Weyhermüller, T., Goswami, U., Mukherjee, C. (2017). A highly stable L-alanine-based mono(aquated) Mn(II) complex as a T1-weighted MRI contrast agent Dalton Transactions 46(31), 10426-10432. https://doi.org/10.1039/c7dt02282d
- Shit, M., Bera, S., Maity, S., Weyhermüller, T., Ghosh, P. (2017). Coordination of o-benzosemiquinonate, o-iminobenzosemiquinonate and aldimine anion radicals to oxidovanadium(IV) New Journal of Chemistry 41, 4564-4572. https://doi.org/10.1039/c7nj00186j
- Levin, N. Perdoménico, J., Bill, E., Weyhermüller, T., Slep, L.D. (2017). Pushing the photodelivery of nitric oxide to the visible: are {FeNO}7 complexes good candidates? Dalton Transactions 46(46), 16058-16064. https://doi.org/10.1039/c7dt03142d
- Shit, M., Bera, S., Maity, S., Maji, S., Weyhermüller, T., Ghosh, P. (2016). Oxidovanadium Complexes of 2,2'-Bipyridine, 1,10 Phenanthroline, and p-Nitro-o-aminophenol - Radical versus Nonradical States European Journal of Inorganic Chemistry 2016(3), 330-338. https://doi.org/10.1002/ejic.201501246
- Bera, S., Maity, S., Weyhermüller, T., Ghosh, P. (2016). Radical non-radical states of the [Ru(PIQ)] core in complexes (PIQ=9,10-phenanthreneiminoquinone) Dalton Transactions 45(19), 8236-8247. https://doi.org/10.1039/c6dt00091f
- Bera, S., Mondal, S., Maity, S., Weyhermüller, T., Ghosh, P. (2016). Radical and Non-Radical States of the [Os(PIQ)] Core (PIQ = 9,10-Phenanthreneiminoquinone): Iminosemiquinone to Iminoquinone Conversion Promoted o-Metalation Reaction Inorganic Chemistry 55(10), 4746-4756. https://doi.org/10.1021/acs.inorgchem.6b00040
- Wang, M., Weyhermüller, T., Bill, E., Ye, S., Wieghardt, K. (2016). Structural and Spectroscopic Characterization of Rhenium Complexes Containing Neutral, Monoanionic, and Dianionic Ligands of 2,2'-Bipyridines and 2,2':6,2''-Terpyridines: An Experimental and Density Functional Theory (DFT)-Computational Study Inorganic Chemistry 55(10), 5019-5036. https://doi.org/10.1021/acs.inorgchem.6b00609
- Dar, U.A., Shand, S., Lande, D.N., Rao, S.S., Patil, Y.P., Gejji, S.P., Nethaji, M., Weyhermüller, T., Salunke-Gawali, S. (2016). Molecular structures of 2-hydroxy-1,4-naphthoqinone derivatives and their zinc(II) complexes: Combining experiment and density functional theory Polyhedron 113, 61 72. https://doi.org/10.1016/j.poly.2016.04.002
- Bhand, S., Patil, R., Shinde, Y., Lande, D.N., Rao, S.S., Kathawate, L., Gejji, S.P., Weyhermüller, T., Salunke-Gawali, S. (2016). Tautomerism in o-hydroxyanilino-1,4-naphthoquinone derivatives: Structure, NMR, HPLC and density functional theoretic investigations Journal of Molecular Structure 1123, 245-260. https://doi.org/10.1016/j.molstruc.2016.06.026
- Kundu, S., Mondal, A., Weyhermüller, T., Sproules, S., Ghosh, P. (2016). Molecular and electronic structures of copper-cuprizone and analogues Inorganica Chimica Acta 451, 23-30. https://doi.org/10.1016/j.ica.2016.06.040
- Maity, S., Kundu, S., Bera, S., Weyhermüller, T., Ghosh, P. (2016). Mixed-Valence o-Iminobenzoquinone and o-Iminobenzosemiquinonate Anion Radical Complexes of Cobalt: Valence Tautomerism European Journal of Inorganic Chemistry 2016(22), 3680-3690. https://doi.org/10.1002/ejic.201600525
- Maity, S., Kundu, S., Bera, S., Weyhermüller, T., Ghosh, P. (2016). o-Iminobenzoquinone and o-Iminobenzosemiquinonate Anion Radical Complexes of Rhodium and Ruthenium European Journal of Inorganic Chemistry 2016(22), 3691-3697. https://doi.org/10.1002/ejic.201600526
- Levin, N., Codesido, N.O., Bill, E., Weyhermüller, T., Gaspari, A.P.S., da Silva, R.S., Olabe, J.A., Slep, L.D. (2016). Structural, Spectroscopic, and Photochemical Investigation of an Octahedral NO Releasing {RuNO}7 Species Inorganic Chemistry 55(16), 7808-7810. https://doi.org/10.1021/acs.inorgchem.6b00719
- Shit, M., Maity, S., Bera, S., Weyhermüller, T., Ghosh, P. (2016). Coordination of o-benzosemiquinonate, o-iminobenzosemiquinonate, 4,4'-di-tert-butyl-2,2'-bipyridine and 1,10-phenanthroline anion radicals to oxidovanadium(IV) New Journal of Chemistry 40(12), 10305-10315. https://doi.org/10.1039/c6nj02220k
- Bera, S., Maity, S., Weyhermüller, T., Ghosh, P. (2016). Arylamino radical complexes of ruthenium and osmium: dual radical counter in a molecule Dalton Transactions 45(48), 19428-19440. https://doi.org/10.1039/c6dt03728c
Chemical synthesis, X-ray structure analysis
Synthesis of Model Compounds
Research in my group focuses on the synthesis of small molecular model complexes for spectroscopic investigations. Systematic variations of structural and electronic features in a series of compounds of similar composition allows to correlate the spectroscopic response with structure. Our samples are typically analyzed by standard methods and their structure determined before they are further investigated (Mössbauer, EPR, SQUID, XAS, etc.). The combination of exact structural data with high- quality spectroscopic measurements of model compounds makes it possible to test and improve quantum chemical methods which in return help to interpret data obtained from catalytically active systems.
Model Systems for Nitrogenase
We have been working on modeling the catalytically active site of nitrogenase for some time. This bacterial enzyme catalyzes the conversion of atmospheric nitrogen to NH3 under physiological conditions. Ammonia is the primary source for the biosynthesis of essential nitrogen compounds in these bacteria and of plants which live in symbiosis with such organisms.
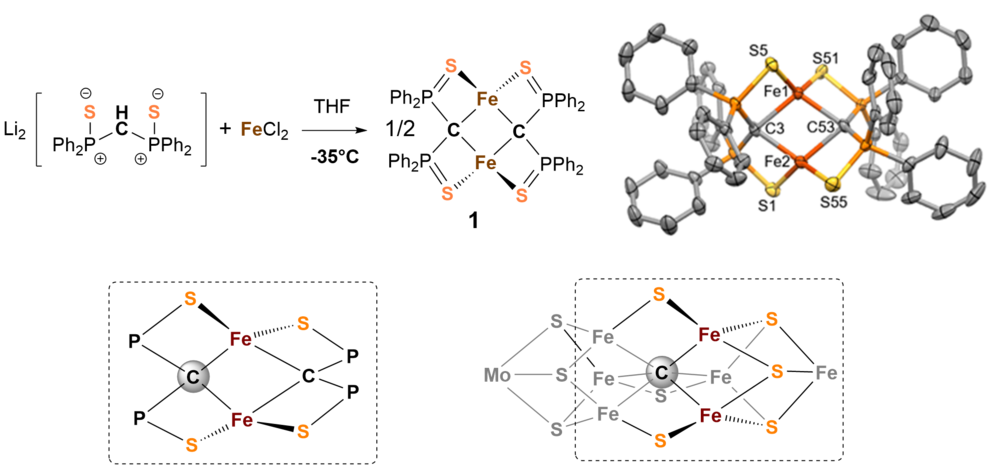
The chemical activation of nitrogen is extremly challenging since the triple bond of molecular nitrogen is the most inert small molecule one could think of. We have learned a lot about the chemistry and structure of nitro- genase but the exact electronic structure in the reaction cycle, the catalytic mechanism and the function of the interstitial carbon atom[1] in the molybdenum cofactor remain unclear. The metallic cofactor FeMoco in nitro- genase has been identified to be the active site of the enzyme where nitrogen binds and ammonia is released. It is basically composed of seven iron-, a molybdenum-center, nine sulfides, and a central carbide ion (see structure of framework in Fig. 1).
We are particulary interested in the chemical function of the carbide ion in the cluster and have therefore tried to synthesize model systems that show similarities to the natural example.
We have investigated a series of carbon bridged dinuclear iron complexes which are accessible by reaction of bis(diphenylthiophosphinoyl)methanediide2- with FeCl2 in THF. [2] Figure 1 shows the composition and structure of complex 1 and demonstrates resemblance with FeMoco.
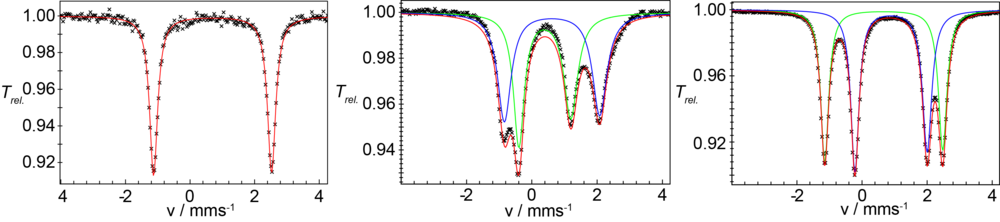
Compound 1 can be reversibly electrochemically or chemi-cally one-electron oxidized to form the mixed-valent Fe(II)Fe(III)-complex 2. Figure 2 shows the zero-field 57Fe-Mössbauer spectra of 1 and 2 which clearly show that the iron centers in the complexes are high-spin Fe(II)Fe(II) and Fe(II)Fe(III) ions, respectively.
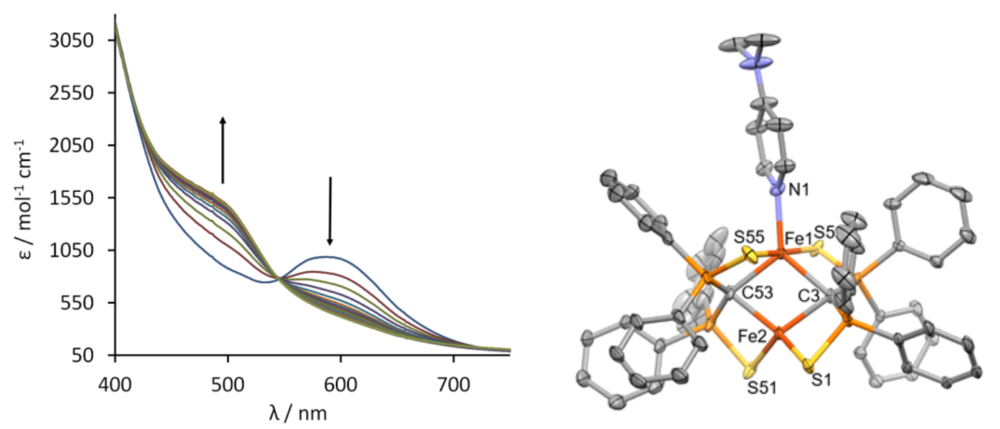
Complex 1 can bind suitable substrates by expansion of the coordination number from 4 to 5 at one iron site. Figure 3 shows the change of the UV/vis spectrum upon addition of dimethylaminopyridine (DMAP). Spectro- metric titration and x-ray structure anaylsis of the product reveal that 1 can exactly bind one molecule DMAP to form compound 3. The 57Fe-Mössbauer spectrum shown in Figure 2 (right) clearly indicates the inequi- valence of the two Fe(II) sites in 3.
X-Ray Structure Determination
Knowledge of the precise three-dimensional structure of the model compounds and catalytically active compounds studied in our departments is of great importance to understand the properties and reactivity of these molecules. The determined structure of the metal complexes also forms the basis of quantum mechanical calculations on these substances, which in turn serve to understand the reactivity and spectroscopic properties that are investigated in the department “Inorganic Spectroscopy” and the "Joint Workspace".
The work in my group focuses on the structure elucidation of small and medium-sized molecules using X-ray diffraction and NMR spectroscopy. Research on mole- cular transition metal compounds for catalysis relies on structure determination by means of X-ray diffraction on single crystals, since self-organization effects often dominate coordination chemistry and targeted synthesis of compounds is only possible to a limited extent. It is often not easily possible to determine the structure using other methods, such as NMR spectroscopy, since high-resolution NMR spectra cannot normally be obtained in the presence of paramagnetic metal centers. Independently of this, the X-ray structure analysis of the single crystal provides highly precise information about the three-dimensional arrangement of atoms and produces precise bond lengths and bond angles, which are of fundamental importance for understanding the chemical properties of a compound. However, the prerequisite for determining the structure using this method are single crystals, which are not always easy to obtain, sometimes not at all. NMR spectroscopy is very well suited for deter- mining the structure in solution. It provides detailed information about the connectivity of atoms in molecules in diamagnetic compounds, but cannot determine exact values for bond lengths and angles. However, it is an excellent and indispensable tool, especially for the rapid assessment of synthetic intermediates and for the characterization of target compounds.
References
[1] a) Lancaster, K. M.; Roemelt, M.; Ettenhuber, P.; Hu, Y.; Ribbe, M. W.; Neese, F.; Bergmann, U.; DeBeer, S., X-ray Emission Spectroscopy Evidences a Central Carbon in the Nitrogenase Iron-Molybdenum Cofactor. Science 2011, 334 (6058), 974. b) Spatzal, T.; Aksoyoglu, M.; Zhang, L.; Andrade, S. L. A.; Schleicher, E.; Weber, S.; Rees, D. C.; Einsle, O., Evidence for Interstitial Carbon in Nitrogenase FeMo Cofactor. Science 2011, 334 (6058), 940-940.
[2] a) Yogendra, S.; Wilson, D.; Hahn, A.; Weyhermüller, T.; van Stappen, C.; Holland, P.; DeBeer, S., Sulfur-Ligated [2Fe-2C]-Clusters as Synthetic Model Systems for Nitrogenase subm. Inorg. Chem. 2022 under review b) Fustier-Boutignon, M.; Heuclin, H.; Le Goff, X. F.; Mezailles, N., Transmetalation of a nucleophilic carbene fragment: from early to late transition metals. Chem. Commun., 2012, 48 (27), 3306-8.