Dr. Saskia Heumann - Carbon Synthesis and Applications
- Dr. Saskia Heumann
- Group leader
- Carbon Synthesis and Applications
- Heterogeneous Reactions
- +49 (0)208 306 - 3701
- saskia.heumann(at)cec.mpg.de
- Room: 657
Vita
Publications
Full publications list | ORCID | ResearcherID
Selected MPI CEC publications
- Song, F., Straten, J. W., Lin, Y.-M., Ding, Y., Schlögl, R., Heumann, S., Mechler, A. K. (2023). Binder-Free N-Functionalized Carbon Electrodes for Oxygen Evolution Reaction. ChemElectroChem, (10) e202201075, pp. 1-10. doi:10.1002/celc.202201075.
- Choudhury, S. H., Ding, Y., Yi, Y., Rohner, C., Frandsen, W., Lunkenbein, T., Greiner, M., Schlögl, R., Heumann, S. (2022). Oxidation Behavior of Glassy Carbon in Acidic Electrolyte. ChemElectroChem, (9): e202200637, pp. 1-7. doi:10.1002/celc.202200637.
- Ristig, S., Poschmann, M., Folke, J., Gomez-Capiro, O., Chen, Z., Sanchez-Bastardo, N., Schlögl, R., Heumann, S., Ruland, H. (2022). Ammonia Decomposition in the Process Chain for a Renewable Hydrogen Supply. Chemie-Ingenieur-Technik, (94), 1-14. doi:10.1002/cite.202200003.
- Lin, Y., Yu, L., Tang, L., Song, F., Schlögl, R., Heumann, S. (2022). In Situ Identification and Time-Resolved Observation of the Interfacial State and Reactive Intermediates on a Cobalt Oxide Nanocatalyst for the Oxygen Evolution Reaction. ACS Catalysis, 12(9), 5345-5355. doi:10.1021/acscatal.1c05598.
- Fan, H., Folke, J., Liu, Z., Girgsdies, F., Ruland, H., Imlau, R., Ruland,H., Heumann, S., Granwehr, J., Eichel,R.- A., Schlögl, R., Frei, E.,Xing, H. (2021) Ultrathin 2D Fe-Nanosheets Stabilized by 2D Mesoporous Silica: Synthesis and Application in Ammonia Synthesis. ACS Appl. Mater. Inerfaces, 13(25), 30187-30197. doi:10.1021/acsami.1c06771
- Ding, Y., Zhang, L., Gu, Q., Spanos, I., Pfänder, N., Wu, K. H., Schlögl, R., Heumann, S. (2021). Tuning of Reciprocal Carbon-Electrode Properties for an Optimized Hydrogen Evolution. ChemSusChem, 14(12), 2547-2553. doi:10.1002/cssc.202100654.
- Lin, Y., Liu, Z., Yu, L., Zhang, G.-R., Tan, H., Wu, K.-H., Song, F., Mechler, A.K., Schleker, P.P.M., Lu, Q., Zhang, B., Heumann, S. (2021). Overall oxygen electrocatalysis on nitrogen‐modified carbon catalysts: identification of active sites and in situ observation of reactive intermediates Angewandte Chemie International Edition, 60,3299–3306 . https://doi.org/10.1002/anie.202012615
- Wu, K.-H., Zhang, Q., Lin, Y., Ali, M.A., Zhao, S., Heumann, S., Centi, G. (2020). Real‐time CO Detection using a Rotating Gold Ring Electrode: A Feasibility Study ChemElectroChem 7(21), 4417-4422. https://doi.org/10.1002/celc.202001263
- Ding, Y., Zhang, P., Xiong, H., Sun, X., Klyushin, A., Zhang, B., Liu, Z., Zhang, J., Zhu, H., Xiao, Z.-A., Heumann, S., Dai, S. (2020). Tuning Regioselective Oxidation toward Phenol via Atomically Dispersed Iron Sites on Carbon Green Chemistry 22(18), 6025-6032. https://doi.org/10.1039/D0GC01717E
- Ding, Y., Greiner, M., Schlögl, R., Heumann, S. (2020). A Metal Free Electrode: From Biomass Derived Carbon to Hydrogen ChemSusChem 13(16), 4064-4048. https://doi.org/10.1002/cssc.202000714
- Park, H., Uluca-Yazgi, B., Heumann, S., Schlögl, R., Granwehr, J., Heise, H., Schleker, P.P.M. (2020). Heteronuclear cross-relaxation effect modulated by the dynamics of N-functional groups in the solid state under 15N DP-MAS DNP Journal of Magnetic Resonance 312, 106688. https://doi.org/10.1016/j.jmr.2020.106688
- Ding, Y., Gu, Q., Klyushin, A., Huang, X., Choudhury, S.H., Spanos, I., Song, F., Mom, R., Düngen, P., Mechler, A.K., Schlögl, R., Heumann, S. (2020). Dynamic carbon surface chemistry: revealing the role of carbon in electrolytic water oxidation Journal of Energy Chemistry 47, 155-159. https://doi.org/10.1016/j.jechem.2019.12.006
- Kraus, P., Massué, C., Heumann, S., Schlögl, R. (2019). Reliable long-term performance assessment of commercial photovoltaic modules tested under field conditions over 5 years Journal of Renewable and Sustainable Energy 11, 063501. https://doi.org/10.1063/1.5128171
- Lin, Y., Liu, Z., Niu, Y., Zhang, B., Lu, Q., Wu, S., Centi, G., Perathoner, S., Heumann, S., Lu, Y., Su, D.S. (2019). Highly Efficient Metal-Free Nitrogen-Doped Nanocarbons with Unexpected Active Sites for Aerobic Catalytic Reactions ACS Nano. https://doi.org/10.1021/acsnano.9b05856
- Park, H., Schleker, P.P.M., Liu, Z., Kowalew, N., Stamm, T., Schlögl, R., Eichel, R.-A., Heumann, S., Granwehr, J. (2019). Insights on Water Interaction at the Interface of Nitrogen Functionalized Hydrothermal Carbons The Journal of Physical Chemistry C 123(41), 25146-25156. https://doi.org/10.1021/acs.jpcc.9b05323
- Ding, Y., Schlögl, R., Heumann, S. (2019). The role of supported atomically distributed metal species in electrochemistry and how to create them ChemElectroChem 6(15), 3860-3877. https://doi.org/10.1002/celc.201900598
- Lin, Y., Lu, Q., Song, F., Yu, L., Mechler, A.K., Schlögl, R., Heumann, S. (2019). Oxygen Evolution Reaction at Carbon Edge Sites: Activity Evolution and Structure‐Function Relationships Clarified by Polycyclic Aromatic Hydrocarbons Angewandte Chemie International Edition 58(26), 8917-8921. https://doi.org/10.1002/anie.201902884
- Gu, Q., Ding, Y., Liu, Z., Lin, Y., Schlögl, R., Heumann, S., Su, D. (2019). Probing the intrinsic catalytic activity of carbon nanotubes for the metal-free oxidation of aromatic thiophene compounds in ionic liquids Journal of Energy Chemistry 32, 131-137. https://doi.org/10.1016/j.jechem.2018.07.004
- Rodenas, T., Beeg, S., Spanos, I., Neugebauer, S., Girgsdies, F., Algara-Siller, G., Schleker, P.P.M., Jakes, P., Pfänder, N., Willinger, M., Greiner, M., Prieto, G., Schlögl, R., Heumann, S. (2018). 2D Metal Organic Framework-Graphitic Carbon Nanocomposites as Precursors for High-Performance O2-Evolution Electrocatalysts Advanced Energy Materials 8(35), 1802404. https://doi.org/10.1002/aenm.201802404
- Lin, Y., Wu, K.-H., Lu, Q., Gu, Q., Zhang, L., Zhang, B., Sheng Su, D., Plodinec, M., Schlögl, R., Heumann, S. (2018). Electrocatalytic Water Oxidation at Quinone-on-Carbon: A Model System Study Journal of the American Chemical Society 140(44), 14717-14724. https://doi.org/10.1021/jacs.8b07627
- Düngen, P., Greiner, M., Böhm, K.H., Spanos, I., Huang, X., Auer, A.A., Schlögl, R., Heumann, S. (2018). Atomically dispersed vanadium oxides on multiwalled carbon nanotubes via atomic layer deposition: A multiparameter optimization Journal of Vacuum Science & Technology A 36(1), 01A126. https://doi.org/10.1116/1.5006783
- Ding, Y., Klyushin, A., Huang, X., Jones, T., Teschner, D., Girgsdies, F., Rodenas, T., Schlögl, R., Heumann, S. (2018). Cobalt Bridged with Ionic Liquid Polymer on Carbon Nanotube for Enhanced Oxygen Evolution Reaction Activity Angewandte Chemie International Edition 57(13), 3514-3518. https://doi.org/10.1002/anie.201711688
- Düngen, P., Schlögl, R., Heumann, S. (2018). Non-linear thermogravimetric mass spectrometry of carbon materials providing direct speciation separation of oxygen functional groups Carbon 130, 614-622. https://doi.org/10.1016/j.carbon.2018.01.047
- Straten, J.W., Schlecker., P., Krasowska, M., Veroutis, E., Granwehr, J., Auer, A.A., Hateba, W., Becker, S., Schlögl, R., Heumann, S. (2018). N-Funtionalized Hydrothermal Carbon Materials using Urotropine as N-Precursor Chemistry - A European Journal 24(47), 12298-12317. https://doi.org/10.1002/chem.201800341
- Gu, Q.Q., Lin, Y.M., Heumann, S., Su, D.S. (2017). Nanocarbons for Catalytic Desulfurization Chemistry - An Asian Journal 12(22), 2876-2883. https://doi.org/10.1002/asia.201700995
- Yi, Y.M., Weinberg, G., Prenzel, M., Greiner, M., Heumann, S., Becker, S., Schlögl, R. (2017). Electrochemical corrosion of a glassy carbon electrode Catalysis Today 295, 32-40. https://doi.org/10.1016/j.cattod.2017.07.013
- Lin, Y.M., Wu, K.H., Yu, L.H., Heumann, S., Su, D.S. (2017). Efficient and Highly Selective Solvent-Free Oxidation of Primary Alcohols to Aldehydes Using Bucky Nanodiamond ChemSusChem 10(17), 3497-3505. https://doi.org/10.1002/cssc.201700968
- Düngen, P., Prenzel, M., Van Stappen, C., Pfänder, N., Heumann, S., Schlögl, R. (2017). Investigation of Different Pre-Treated Multi-Walled Carbon Nanotubes by Raman Spectroscopy Materials Sciences and Applications 8, 628-641. https://doi.org/10.4236/msa.2017.88044
- Hävecker, M., Düngen, P., Buller, S., Knop-Gericke, A., Trunschke, A., Schlögl, R. (2017). Restructuring of silica supported vanadia during propane oxidative dehydrogenation studied by combined synchrotron radiation based in situ soft X-ray absorption and photoemission Catalysis Structure and Reactivity 3, 104-111. https://doi.org/10.1080/2055074X.2017.1287535
- Buller, S., Strunk, J. (2016). Nanostructure in energy conversion Journal of Energy Chemistry 25(2), 171-190. https://doi.org/10.1016/j.jechem.2016.01.025
- Buller, S., Heise-Podleska, M., Pfänder, N., Willinger, M., Schlögl, R. (2016). Carbon nanotubes as conducting support for potential Mn-oxide electrocatalysts: Influences of pre-treatment procedures Journal of Energy Chemistry 25(2), 265-271. https://doi.org/10.1016/j.jechem.2016.01.022
Group members
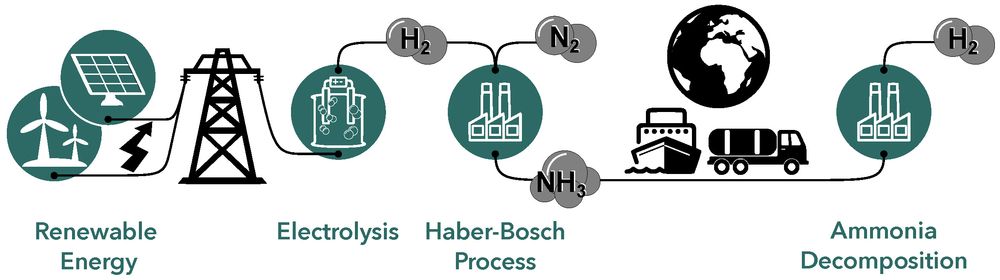
The aim of our research is the knowledge-based development of advanced catalysts and their application in different energy-related processes. In the process chain that enables our future energy supply, based on hydrogen (H2) as chemical energy carrier, numerous catalysts are in use. The process chain comprises the generation of renewable energies (in sun and wind-rich regions), the use of electricity in electrolysers for the production of hydrogen, its conversion in the Haber-Bosch process with nitrogen (N2) into ammonia (NH3) on site, global transport and the recovery of the hydrogen by ammonia decomposition at the desired destination.

Part of the work focuses on electrode materials mainly for the oxygen evolution reaction (OER) in water electrolysis. We are working on carbon and non-noble metal based materials. The carbon synthesis strategy of the group is based on the use of molecular precursors and controllable condensation reactions in liquid phase (hydrothermal synthesis). Hydrocarbons like glucose are used for this bottom-up approach to obtain solid hydrothermal carbons. For large-scale productions, biomass can later be used as cheap and abundant carbon source. The incorporation of oxygen or nitrogen functional groups can be tuned by varying the initial synthesis pH or by the addition of nitrogen containing precursor like for example urotropine. [1] The distribution of the oxygen functional groups, as well as the morphology of the carbonaceous product, is controlled by process parameters. The high number of functional groups leads to intrinsic binding properties that allow the preparation of functional disc electrodes by pressing and thermal annealing. [2] The macroscopic dimension of the bulk electrode allows quantitative analytical investigations before, after and during the electrochemical testing.
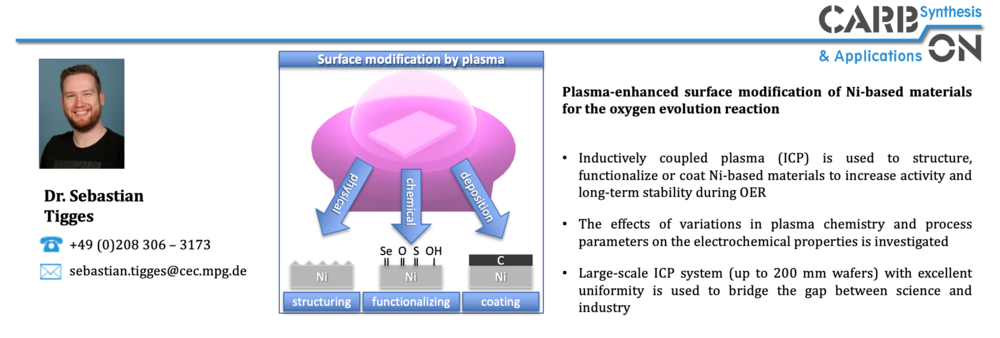
The targeted surface modification of materials by means of a post-synthesis plasma treatment is also investigated to produce non-equilibrium phases with improved electrocatalytic performance. Plasma treatment provides a cost-effective and scalable means for process-independent optimization of materials. Plasma treatment also offers the advantage that it can be integrated relatively easily and cost-effectively into existing large-scale processes, for example roll-to-roll processes. The focus here is on Ni-based materials, which are being investigated as part of the PrometH2eus project, which is part of the H2Giga lead project funded by the German Federal Ministry of Education and Research (BMBF).
Systematic, detailed and in-situ and in-operando investigations of the electrode materials allow us to gain insights into structure-property correlations that enables us to improve the performance at a knowledge-based level. [3, 4, 5] The long-term stability is an important factor, as well as the interaction of the electrode surfaces with the electrolyte and impurities therein, which are also investigate in detail. [6, 7, 8]

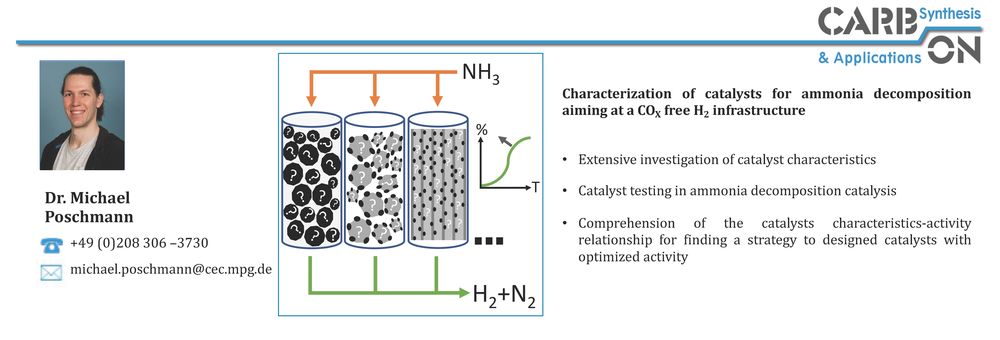
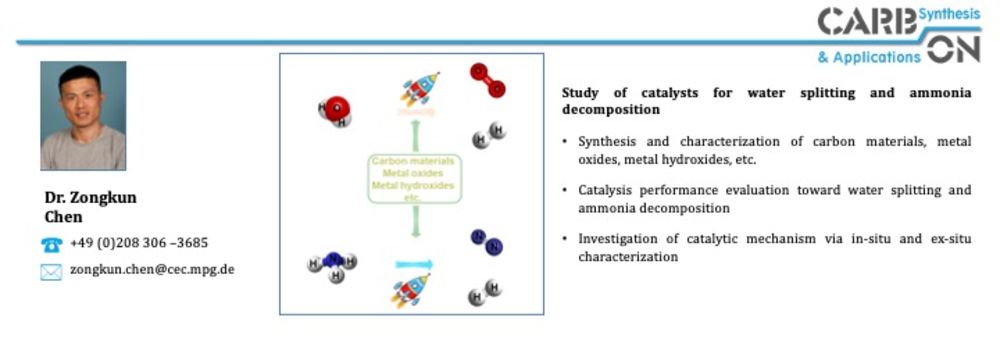
In the area of ammonia decomposition, the influence of carbon support materials on catalytic activity has been recognized. Nitrogen-doped carbons used as support materials for NH3 decomposition at low temperatures have shown an advantage over undoped supports according to several reports. Higher catalytic activities for NH3 decomposition at 450 °C for Ru on a nitrogen-doped ordered mesoporous carbon compared to Ru on an undoped OMC (onion like carbon), CNT (carbon nanotube) and AC (activated carbon) have been reported. However, in addition to the beneficial metal-support interactions, the basicity of the nitrogen-doped support has also been described as a "basic promoter" for Ru. Basic promoters, such as the alkali metal Cs, increase the catalytic activity of Ru as a catalyst in the NH3 decomposition reaction. Hydrothermal carbonization (HTC) is a well-known process for the synthesis of carbonaceous materials, which has been demonstrated as a potential route for developing tunable carbon materials from sustainable resources as described above. HTC is performed at low reaction temperatures (T ≤ 200 °C) and allows doping with heteroatoms and tuning of the degree of graphitization by thermal treatment at higher temperatures. Within the AmmoRef research network in the TransHyDE lead project, which is funded by the German Federal Ministry of Education and Research (BMBF), carbon-based catalysts for ammonia reforming are synthesized and investigated and developed in the project network.
Selected Paper
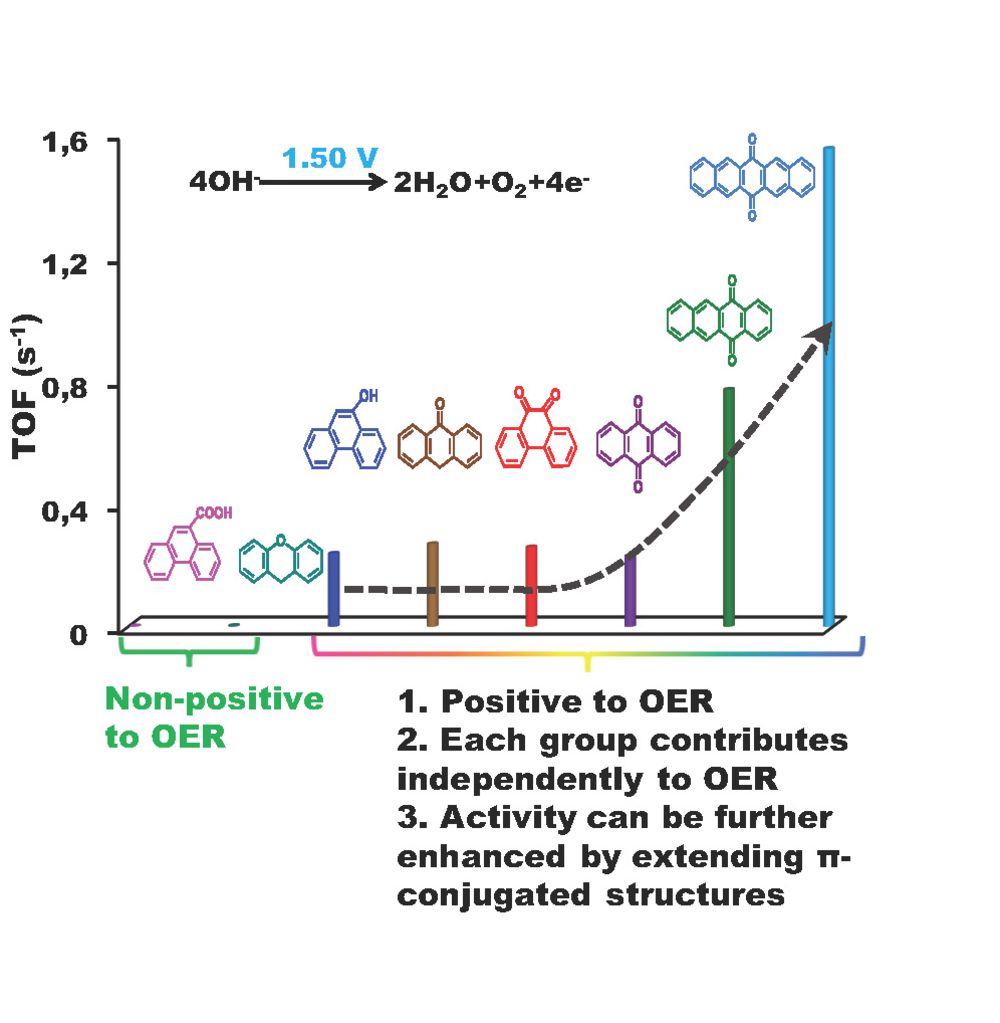
Model structures were decorated onto typical nanocarbon surfaces like onion-like carbons (OLC) or multiwalled carbon nanotubes (MWCNT) to build aromatic molecule-modified carbon systems. We show that edge (including zigzag and armchair) quinones in a conjugated π network are the true active centers, and the roles of ether and carboxyl groups are excluded in the OER process. The plausible rate-determining step could be singled out by H/D kinetic isotope effects. The turnover frequency per C=O (∼0.323 s−1 at η = 340 mV) in 0.1 M KOH and the optimized current density (10 mA/cm2 at 1.58 V vs RHE) of quinone-modified carbon systems are comparable to those of promising metal-based catalysts.
DOI: 10.1021/jacs.8b07627 J. Am. Chem. Soc. 2018, 140, 14717−14724
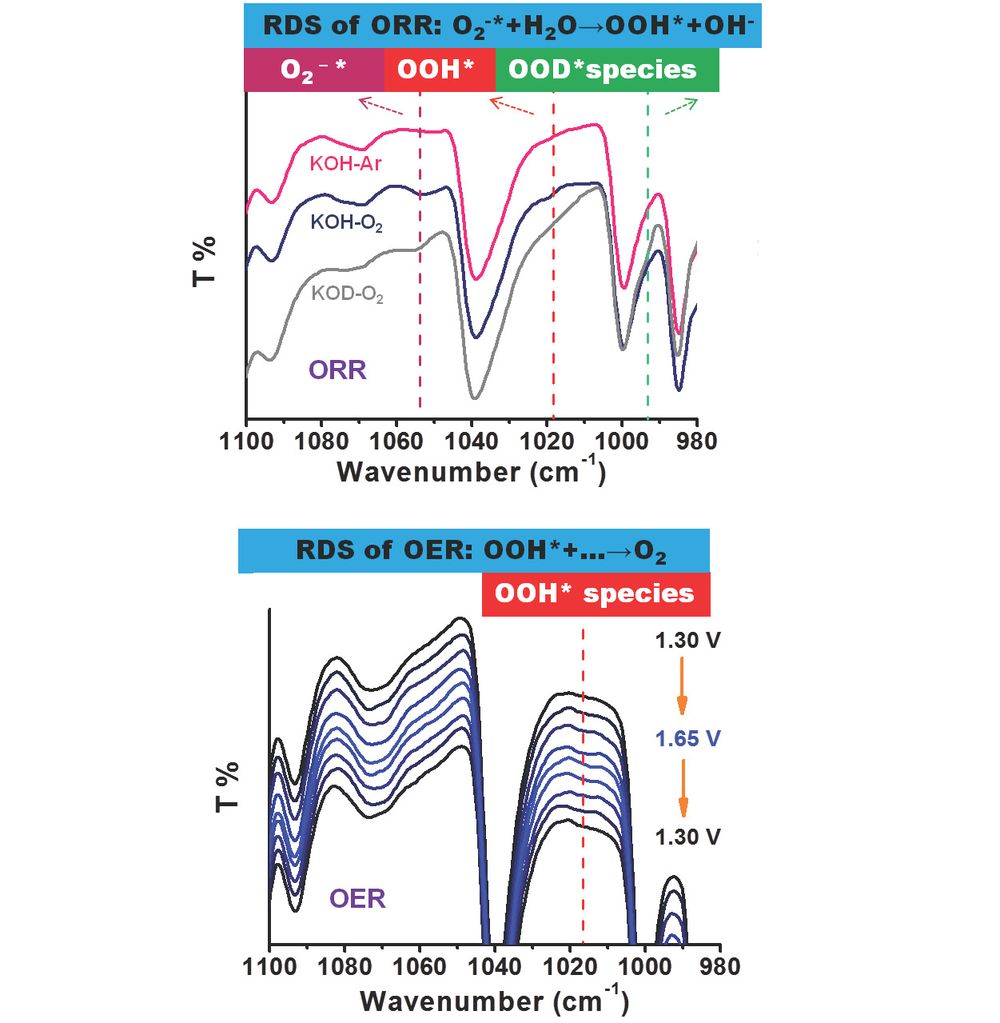
The recent mechanistic understandings including active sites, adsorbed intermediate products and rate-determining steps (RDS) of nitrogen (N)-modified carbon catalysts in electrocatalytic oxygen reduction (ORR) and oxygen evolution reaction (OER), however, are still rife with controversies due to the inevitable coexistence of diverse N configurations and the technical limitation for observing formed intermediates. In this work, seven kinds of aromatic molecules with designated single N species are used as model structures to investigate the explicit role of each common N group in both ORR and OER. Specifically, dynamic evolution of active sites and key adsorbed intermediate products including O2 (ads), superoxide anion O2-* and OOH* are monitored with in situ spectroscopy. We propose that the formation of *OOH species from O2-* (O2-*+H2O→OOH*+OH-) is a possible RDS during the ORR process, whereas the generation of O2 from OOH* species is the most possible RDS during the OER process.
DOI: 10.1002/anie.202012615 Angew. Chem. Int. Ed. 2021, 60 3299-3306
DOI: 10.1002/ange.202012615 Angew. Chem. 2021, 133, 3336-3343
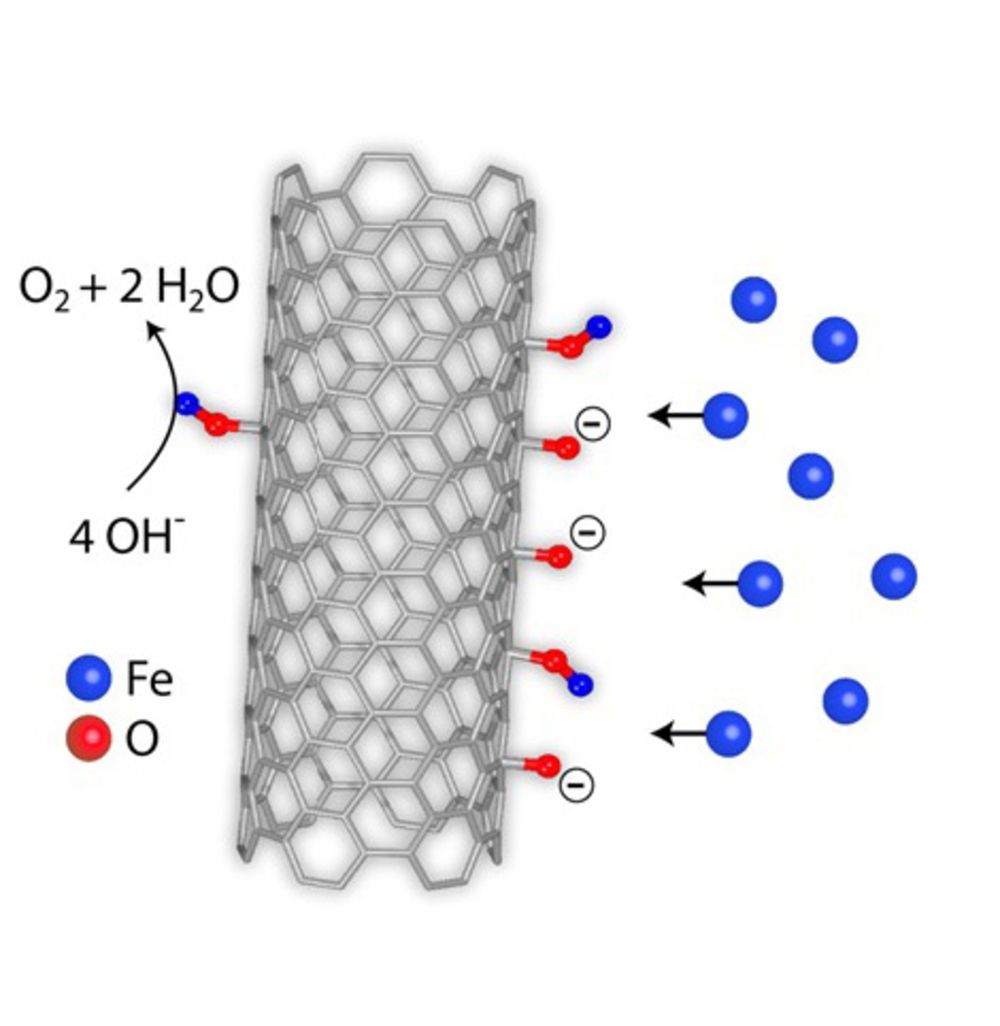
Carbon materials have been widely used as electrodes, but the mechanistic roles are still not clear due to the complexity of the carbon surface chemistry. Herein we clarify that intrinsic material properties of carbon have to be activated by extrinsic factors. Pure carbon has no catalytic activity when used as electrode for electrocatalytic water oxidation. The evolution of oxygen functional groups on the carbon surface with increasing potential and the subsequent formation of real active sites with iron impurities from the electrolyte have been confirmed. These in-situ formed active sites protect the carbon from deep oxidation. This unprecedented finding not only provides insight into the dynamic evolution of carbon electrode surface chemistry and raises awareness of the need for detailed surface analysis under operando conditions, but also suggests a direction for the development of scalable and high- performance carbon-based electrode systems for various electrochemical applications.
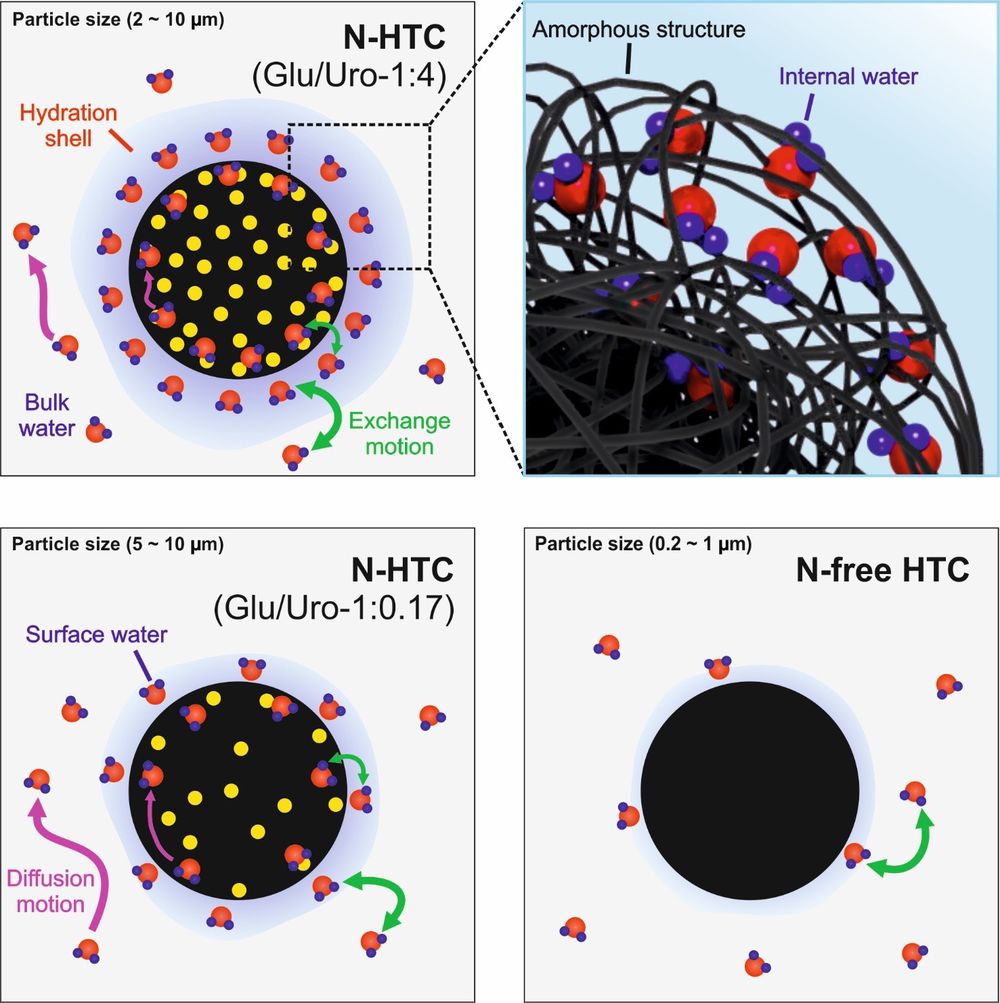
Solid-state nuclear magnetic resonance (NMR), longitudinal (T1) relaxation time, and diffusion NMR were employed to investigate the structure and water dynamics for HTC and nitrogen-functionalized hydrothermal carbon (N-HTC) samples ((N)-HTC). Results showed that the presence of N-functional groups influences the water interaction with (N)-HTC more strongly than surface area, pore size distribution, or oxygenated functional groups. Furthermore, the degree of water interaction can be tuned by adjusting the synthesis temperature and the precursor ratio. Water motion was more strongly inhibited in N-HTC than in N-free HTC, thereby suggesting the existence of a differently structured hydration shell around N-HTC particles. In addition, the diffusion data of water in the N-HTC material show two components that do not exchange on the time scale of the experiment (tens of milliseconds), indicating a significant fraction of slow mobile water that exists inside the structure of N-HTC. 1H–2H isotope exchange and cross-polarization NMR results show this internal water only in a near-surface layer of the N-HTC particles. Based on these findings, a model for water interaction with (N)-HTC particles is proposed.
DOI: 10.1021/acs.jpcc.9b05323 J. Phys. Chem. C 2019, 123, 25146−25156
References
[1] Straten, J.W., Schleker, P., Krasowska, M., Veroutis, E., Granwehr, J., Auer, A.A., Hetaba, W., Becker, S., Schlögl, R., and Heumann, S., (2018). N-Functionalized Hydrothermal Carbon Materials using Urotropine as N-Precursor, Chemistry - A European Journal, 24, 12298-12317. https://doi.org/10.1002/chem.201800341
[2] Ding, Y., Zhang, L., Gu, Q., Spanos, I., Pfander, N., Wu, K.H., Schlogl, R., and Heumann, S. (2021). Tuning of Reciprocal Carbon-Electrode Properties for an Optimized Hydrogen Evolution, ChemSusChem. https://doi.org/10.1002/cssc.202100654
[3] Lin, Y., Wu, K.-H., Lu, Q., Gu, Q., Zhang, L., Zhang, B., Su, D., Plodinec, M., Schlögl, R., and Heumann, S., (2018). Electrocatalytic Water Oxidation at Quinone-on-Carbon: A Model System Study, JACS, 140 (44), 14717-14724. https://doi.org/10.1021/jacs.8b07627
[4] Lin, Y., Lu, Q., Song, F., Yu, L., Mechler, A.K., Schlögl, R., and Heumann, S., (2019). Oxygen Evolution Reaction at Carbon Edge Sites: Investigation of Activity Evolution and Structure-Function Relationships with Polycyclic Aromatic Hydrocarbons / Sauerstoffentwicklungsreaktion an Kohlenstoffkanten: Aktivitätsentwicklung und Struktur‐Eigenschafts‐Beziehungen, untersucht anhand polyzyklischer aromatischer Kohlenwasserstoffe, Angew. Chem. Int. Ed. / Angew. Chem., 58 / 131 (26), 8917-8921 / 9010-9014. https://doi.org/10.1002/anie.201902884, https://doi.org.10.1002/ange.201902884
[5] Ding, Y., Klyushin, A., Huang, X., Jones, T., Teschner, D., Girgsdies, F., Rodenas, T., Schlogl, R., and Heumann, S., (2018). Cobalt-Bridged Ionic Liquid Polymer on a Carbon Nanotube for Enhanced Oxygen Evolution Reaction Activity / Ein aktiver und stabiler Cobaltkatalysator für die Sauerstoffentwicklungsreaktion: Polymerisation einer ionischen Flüssigkeit, Angew. Chem. Int. Ed. / Angew. Chem., 130 / 57, 3573-3577 / 3514-3518. https://doi.org/10.1002/anie.201711688, https://doi.org/10.1002/ange.201711688
[6] Yi, Y., Weinberg, G., Prenzel, M., Greiner, M., Heumann, S., Becker, S., and Schlögl, R., (2017). Electrochemical corrosion of a glassy carbon electrode, Catalysis Today, 295, 32-40. https://doi.org/10.1016/j.cattod.2017.07.013
[7] Ding, Y., Gu, Q.Q., Klyushin, A., Huang, X., Choudhury, S.H., Spanos, I., Song, F.H., Mom, R., Dungen, P., Mechler, A.K., Schlogl, R., and Heumann, S. (2020). Dynamic carbon surface chemistry: Revealing the role of carbon in electrolytic water oxidation, Journal of Energy Chemistry, 47: p. 155-159. https://doi.org/10.1016/j.jechem.2019.12.006
[8] Park, H., Schleker, P.P.M., Liu, Z., Kowalew, N., Stamm, T., Schlögl, R., Eichel, R.-A., Heumann, S., and Granwehr, J., (2019). Insights into Water Interaction at the Interface of Nitrogen-Functionalized Hydrothermal Carbons, The Journal of Physical Chemistry C, 123, 25146-25156. https://doi.org/10.1021/acs.jpcc.9b05323
Alumni
| Dr. Sylvia Becker | Dr. Yangming Lin | Dr. Jan Willem Straten |
| Dr. Sakeb Hasan Choudhury | Dr. Zigeng Liu | Fabian Wachholz |
| Dr. Yuxiao Ding | Dr. Heeyong Park | Dr. Shuchang Wu |
| Dr. Pascal Düngen | Dr. Marina Prenzel | Dr. Youngmi Yi |
| Dr. Guillermo Alvarez Ferrero | Dr. Tania Rodenas | Dr. Linhui Yu |
| Dr. Qingqing Gu | Dr. Philipp Schleker | |