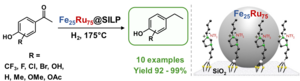
Substituted ethylphenols are of great interest as they are important building blocks for the production of pharmaceuticals, fine chemicals and polymers. However, the synthesis of these compounds still remains challenging. In particular, the commonly used acidic hydrodeoxygenation catalysts entail undesired side reactions.
In a recent Chemical Communications publication, Dr. Alexis Bordet (Multifunctional Catalytic Systems) and co-workers reported a new system capable of performing the hydrodeoxygenation reaction of hydroxyacetophenone derivatives to ethylphenol derivatives, with high selectivity and activity for a broad spectrum of substrates.
The research in the Bordet group focuses on the design and synthesis of catalytic systems based on metallic nanoparticles immobilized on molecularly modified supports. In their current approach, the scientists utilized bimetallic iron ruthenium nanoparticles immobilized on an imidazolium-based supported ionic liquid phase (Fe25Ru75@SILP). Importantly, this non-acidic catalytic system avoids the formation of side-products and conserves the aromatic moiety. Lisa Goclik, a PhD Student in the group and first author of this publication, stresses the importance of the broad scope of substrates used in this study: “All the hydroxyacetophenone derivatives considered were effectively converted, providing ethylphenols in excellent yields and selectivities and thus demonstrating the wide applicability of the Fe25Ru75@SILP catalytic system as can be also seen in the presented figure.” Another significant aspect of this study is that some of these substrates can be obtained from renewable resources, thus opening the way for more sustainable products. “Moreover, a successful recycling of the catalyst was performed, paving the way towards its testing under industrially relevant conditions”, adds the scientist.
Original Publication: Goclik, L., Offner-Marko, L., Bordet, A., Leitner, W. (2020). Selective Hydrodeoxygenation of Hydroxyacetophenones to Ethyl-Substituted Phenol Derivatives Using a FeRu@SILP Catalyst Chemical Communications 56(66), 9509‑9512. DOI: 10.1039/D0CC03695A