Advancing Sustainable Hydrogen Peroxide Production
Hydrogen peroxide (H2O2) is an environmentally friendly oxidant with a wide range of applications in the industrial and medical sectors. The global demand for H2O2 is expected to reach 5.7 million tons annually by 2028 so searching for sustainable green production methods increasingly important. Electrochemical H2O2 synthesis offers a promising alternative to traditional industrial processes, with the potential for decentralized on-site production driven by renewable energy. In a recent study, a team of researchers from MPI CEC and Universität Duisburg-Essen, lead by Viktor Čolić, Max Planck Research Group Leader at MPI CEC, examined the electrochemical processes behind H2O2 generation on carbon electrodes, shedding light on how structural factors like defects, oxygen functional groups, and the presence of alkali metals influence production efficiency and selectivity.
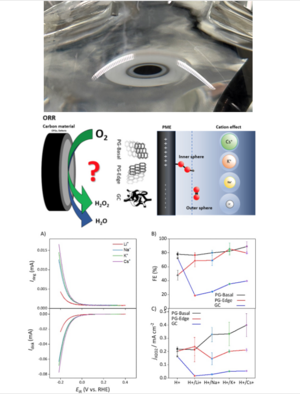
The Role of Carbon-Based Materials in H2O2 Electrosynthesis
While efficient electrocatalysts in H2O2 production based on metal alloys such as Au and Ag-Hg alloys are well-known, their high cost and potential toxicity make large-scale applications challenging. In contrast, carbon-based materials offer an affordable, metal-free alternative. Their defect-rich structures and oxygen functional groups (OFGs), play a crucial role in the electrochemical behavior of H2O2 electrosynthesis. By using PG-edge, PG-basal, and GC as model electrodes, the study explored how these carbon materials, with varying degrees of defect density and OFGs, influence the efficiency and selectivity of the oxygen reduction reaction (ORR) in acidic conditions.
Understanding the Electrochemical Mechanisms
The study highlights two distinct pathways for H2O2 production on carbon electrodes. The PG-basal electrodes predominantly follow an inner-sphere mechanism, while the GC electrode favors an outer-sphere pathway. Despite these differences, both electrodes demonstrated high Faradaic efficiency (FE) for H2O2 generation, underlining the versatility of carbon-based materials in achieving similar efficiency through different reaction mechanisms. Furthermore, the study explores the potential of maximal entropy (PME) on these materials, a key factor that governs the electrochemical interface and influences the selectivity and activity toward H2O2 production.
Influence of Alkali Cations on Selectivity
The study also investigated the role of alkali cations - Li+, Na+, K+, and Cs+ in shaping the reaction pathway and selectivity of the ORR. It was found that large cations, such as Cs+, enhanced the selectivity of PG-basal electrodes toward H2O2 production, while GC electrodes showed a tendency to favor water formation. This phenomenon is attributed to the way alkali cations interact with the carbon electrode surface and affect the electrochemical environment, particularly the binding of reaction intermediates. These findings suggest that adjusting electrolyte composition, particularly alkali ion content, can be a powerful tool for tuning the selectivity of carbon-based electrocatalysts.
Implications for Future Catalysts and Sustainable Chemistry
The insights gained from this study offer significant contributions to the development of carbon-based catalysts for electrochemical H2O2 production. Understanding the relationship between surface defects, oxygen functional groups, and alkali cations opens the door to the rational design of more efficient, metal-free electrocatalysts. This research paves the way for the widespread adoption of carbon-based materials in sustainable chemical processes, promoting greener, more cost-effective solutions for H2O2 production. The continued exploration of these electrochemical mechanisms will be crucial in advancing the field of sustainable chemistry and catalysis.
The open access paper was recently published in the Journal ACS Catalysis.
Original Paper: André Olean-Oliveira, Najeeb Hasnain, Ricardo Martínez-Hincapié, Ulrich Hagemann, Adarsh Jain, Doris Segets, Ioannis Spanos, and Viktor Čolić. Electrochemical Insights into Hydrogen Peroxide Generation on Carbon Electrodes: Influence of Defects, Oxygen Functional Groups, and Alkali Metals in the Electrolyte ACS Catalysis 2024 14 (23), 17675-17689 DOI: 10.1021/acscatal.4c04734 Link